b Fangchenggang Marine Environmental Monitoring and Forecasting Center/Beilun Estuary National Nature Reserve Administration, Fangchenggang 538001, China;
c Guangdong No. 2 Provincial People's Hospital, Guangzhou 510000, China;
d Guangxi Key Laboratory of Marine Environmental Science, Guangxi Academy of Sciences, Nanning 530007, China;
e Key Laboratory of Plant Resource Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China;
f Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China
Harmful algal blooms (HABs) are reported to occur worldwide,which have been a serious problem for aquaculture and fisheries operation and have bad impacts on human health [1]. The blooms of Phaeocystis globosa have frequently occurred along coastal waters and exert serious impacts on ecological environments by releasing toxic hemolytic substances,forming nuisance foam,and causing oxygen depletion [2]. Due to these severe negative effects,several studies have described methods of controlling the bloom of P. globosa [3, 4].
However,the investigation on the chemical constituents of wild P. globosa and its biological activities are not reported yet. In the course of our phytochemical studies on this specimen,two new compounds,namely,taenialactam C and globorin A (1 and 2),as well as six known compounds,cornoside (3) [5],2-phenylethyl-b- D-glucoside (4) [6],3-isopropyl-5-acetoxycyclohexene-2-one-1 (5) [7],4-methylphenol (6) [8],5-[(2S)-2-aminobutyl]-2-methylphenol (7) [9],and 1-(4-methylphenyl)-1-propanone (8) [10] (Fig. 1) were isolated from P. globosa. We also report toxicity properties of the eight compounds against the brine shrimp Artemia salina and juvenile Epinephelus akaara fish.

|
Download:
|
| Fig. 1.Structures of compounds 1–8 isolated from wild P. globosa. | |
2.1. General experimental procedures
UV spectra were recorded in MeOH on a Perkin-Elmer Lambda 35 UV-vis spectrophotometer. The IR spectra were measured in KBr on a WQF-410 FT-IR spectrophotometer. NMR spectra were recorded on a Bruker AV 600 MHz NMR spectrometer with TMS as an internal standard. HRESIMS data were obtained from a Bruker Maxis mass spectrometer. HPLC was performed on a Waters-2695 HPLC system,using a SunfireTM C18 column (150 × 10 mm i.d.,10 μm) coupled to a Waters 2998 photodiode array detector. Optical rotation data were measured by Perkin-Elmer Model 341 polarimeter. The silica gel GF254 used for TLC was supplied by the Qingdao Marine Chemical Factory,Qingdao,China. Spots were detected on TLC under UV light or by heating after spraying with 5% H2SO4 in EtOH. All solvent ratios are measured v/v.
2.2. Plant materialWild P. globosa was collected from the entry of Beilun River,Fangchenggang city,Guangxi province,China,in December 2012. The specimen was identified by Professor Songhui Lv from Jinan University. A voucher specimen (2012-GXAS-0015) was deposited in Guangxi Key Laboratory of Marine Environmental Science,Guangxi Academy of Sciences,China.
2.3. Extraction and isolationP. globosa (35 L) was exhaustively extracted with EtOH (95%,10 L) at 25 ℃ for 3 × 4 days. The solvent was evaporated in vacuo to afford a syrupy residue (45 g) that was suspended in distilled water (0.5 L) and fractionated successively with petroleum ether (3 × 0.5 L),ethyl acetate (3 × 0.5 L),and n-butanol (3 × 0.5 L). The ethyl acetate soluble portion (3.36 g) was subjected to column chromatography on silica gel,using CHCl3-Me2CO (from 10:0 to 0:10) as eluent,giving eleven fractions (A-K). Fraction C was subjected to column chromatography to afford ten sub-fractions (C1-C10). Sub-fraction C3 was subjected to Sephadex LH-20 column chromatography with CHCl3-MeOH (1:1),then separated by HPLC,using the mixtures of MeOH-H2O (35:65) to yield 5 (5.3 mg). Fraction D was subjected to column chromatography to afford eight sub-fractions (D1-D8). Sub-fraction D2 was subjected to Sephadex LH-20 column chromatography with CHCl3-MeOH (1:1),then separated by HPLC,using the mixtures of MeOH-H2O (70:30) to yield 1 (2.7 mg). Fraction F was subjected to column chromatography to afford ten sub-fractions (F1-F10). Sub-fraction F3 was subjected to Sephadex LH-20 column chromatography with CHCl3-MeOH (1:1),then separated by HPLC,using the mixtures of MeOH-H2O (60:40) to yield 8 (0.4 mg) and 3 (0.6 mg),respectively. Fraction H was subjected to column chromatography to afford ten sub-fractions (H1-H10). Sub-fraction H9 was subjected to Sephadex LH-20 column chromatography with CHCl3-MeOH (1:1),then separated by HPLC,using the mixtures of MeOH-H2O (15:85) to yield 7 (1.2 mg). The n-butanol soluble portion (7.83 g) was subjected to column chromatography on silica gel,using CHCl3-Me2OH (from 10:0 to 0:10) as eluent,giving seven fractions (Ba-Bg). Fraction Bf was subjected to column chromatography to afford ten sub-fractions (Bf1-Bf10). Sub-fraction Bf3 was subjected to Sephadex LH-20 column chromatography with CHCl3-MeOH (0:100),then separated by HPLC,using the mixtures of MeOH-H2O (10:90) to yield 2 (4.9 mg). Sub-fraction Bf4 was subjected to Sephadex LH-20 column chromatography with CHCl3-MeOH (0:100),then separated by HPLC,using the mixtures of MeOHH2O (25:75) to yield 4 (1.8 mg). Fraction Bg was subjected to column chromatography to afford five sub-fractions (Bg1-Bg5). Sub-fraction Bg3 was subjected to Sephadex LH-20 column chromatography with CHCl3-MeOH (0:100),then separated by HPLC,using the mixtures of MeOH-H2O (30:70) to yield 6 (1.2 mg).
Taenialactam C (1): White solid; CD (MeOH) Δε220nm + 4.58; 1H NMR (CDCl3,600 MHz) and 13C NMR (CDCl3,150 MHz),see Table 1; HRESIMS m/z 230.1549 (calcd. for C15H19NO + H,230.1539).
|
|
Table 1 1H NMR and 13C NMR data of 1 and 2 (δ in ppm, J in Hz). |
Globorin A (2): Yellow oil; CD (MeOH) Δε215nm + 6.2; 1H NMR (CD3OD,600 MHz) and 13C NMR (CD3OD,150 MHz),see Table 3; HRESIMS: m/z 259.1298 (calcd. for C14H20O3 + Na,259.1305).
|
|
Table 2 Effect of compounds 1–8 on the survival of brine shrimp A. salina. |
|
|
Table 3 Effect of compounds 1–8 on the survival of the juvenile E. akaara fish. |
The toxicity test was conducted with brine shrimp A. salina and grouper E. akaara. The procedures of the toxicity tests followed those described by the US Environmental Protection Agency for toxicity tests for aquatic organisms [11].
The shrimp lethality of the isolates was assessed following the reported method [12, 13, 14]. Brine shrimp eggs (Ocean Star International,Inc.,USA) were hatched in a large beaker of natural seawater (South China Sea),then incubated at room temperature for 48 h. With the help of a light source,the larvae were attracted to one side of the vessel and easily collected for the assay. Compounds 1-8 dissolved in dimethyl sulfoxide (DMSO) at a concentration of 50 mg/mL were diluted in 96-well plates with 200 mL seawater for testing at the final concentrations of 5,50,and 500 mg/mL. Each test was conducted in triplicate with approximately 10 larvae,which were counted under a magnifying glass after 24 h incubation. The controls were prepared in the same manner except that the test samples were omitted. The lethality of larvae was recorded and used for calculating the LC50 with the Lanyu LC50 analysis program (ver. 1.01).
The grouper lethality of the isolates was assessed following the reported method [11]. The grouper E. akaara (less than 48 h old) were obtained from Guangxi Academy of Fishery Sciences and were maintained in the laboratory in artificial seawater at 18 ℃ for 3 d before testing. Triplicate test vessels with 200 mL seawater containing ten juvenile fish were used with test samples. The experimental conditions,including temperature,dissolved oxygen,and pH were kept constant. Mortality was recorded at 12 h,24 h,48 h,and 72 h,and dead juveniles were discarded.
3. Results and discussionTaenialactam C (1) was isolated as a white solid. The molecular formula of 1 was assigned as C15H19NO on the basis of its HR-ESIMS (found inm/z 230.1549 [M+H]+,calcd. for C15H20NO,230.1539) and NMR data,implying seven double bond equivalents. The NMR data of 1 revealed the presence of one methyl group,four sp3 methylene groups,two sp2 methylene groups,three sp3 methine groups,and five quaternary sp2 carbon atoms (Table 1). The presence of an α-methyl-α,β-unsaturated-γ-lactam moiety was indicated by the carbon atom resonances at δC 174.8 (C-12),157.2 (C-7),126.6 (C-11),61.5 (C-8),and 8.0 (13-CH3) [15]. Two additional unsaturated functionalities were indicated by 13C NMR resonances of δC 156.3 (C-4),145.5 (C-10),114.7 (C-14),and 107.1 (C-15),indicating the presence of two disubstituted olefinic double bonds. The above information,in combination with the molecular formula,showed the molecule to have a tricyclic skeleton.
1H-1H-COSY and HMBC correlations (Fig. 2) were used to establish the molecular skeleton of 1. Spin systems were revealed by analysis of COSY correlations corresponding to the H-6/H-5/H- 1/H-2/H-3 and H-8/H-9. These data,together with the key HMBC correlations from H-14 to C-3,C-4,and C-5; H-6 to C-1,C-4,C-5,C- 7,C-8,and C-11; Me-13 to C-7,C-8,and C-12; H-9 to C-1 and C-8; H-15 to C-1 and C-9 in combination with the δ values of C-8 and C- 12 showed the presence of a guaiane sesquiterpenoid skeleton with an α-methyl-α,β-unsaturated-γ-lactammoiety [15] and two olefinic double bonds,that is between C-4 and C-14; C-10 and C- 15.

|
Download:
|
| Fig. 2.Key 1H–1H-COSY correlations and HMBCs (H→C) of 1 and 2. | |
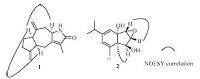
The relative configuration of 1 was proposed on the basis of key NOESY correlations (Fig. 3). The observation of NOESY correlation between the H-1 and H- 5; H-1 and H-8 allowed assignment of the relative stereochemistries of H-1,H-5,and H-8 in the β-orientation. A NOESY correlation between Hβ-C(9) and H-8 was also observed. The coupling constants between H-9 and H-8 (J9β,8 = 14.3 Hz,J9α,8 ≤ 1 Hz) required a dihedral angle between H-8 and Hα-9 close to 908 and of almost 1808 between H-8 and Hβ-9. All these geometric constraints dictated by the observed NOESY correlations and coupling constants are compatible with H-8 having the β-orientation.
|
Download:
|
| Fig. 3.The key NOESY correlations of 1 and 2. | |
Finally,the absolute stereo-structure of 1 was determined by the circular dichroic (CD) spectrum on a,b-unsaturated γ-lactam moiety [15, 16]. Namely,the CD spectrum of 1 showed the characteristic Cotton curve (Δε + 4.58,at 220 nm) for the 8S configuration in endo-α,β-unsaturated lactams. On the basis of above analysis,the structure of 1 was thus established as depicted in Fig. 1.
Globorin A (2),was purified as a yellow oil with the molecular formula C14H20O3 as determined by HRESIMS (found [M+Na]+ at m/z 259.1298,calcd. [M+Na]+,259.1305) as well as 1H NMRand 13C NMR spectroscopic data (Table 1),implying five double bond equivalents. The NMR data of 2 revealed the presence of four methyl groups,four sp3 methine groups,two sp2 methine groups,and two quaternary sp3 carbon atoms (Table 1). An additional unsaturated functionality was indicated by 13C NMR resonances of δC 152.4 (C-6),147.5 (C-10),129.7 (C-7),and 119.5 (C-8),indicating the presence of two trisubstituted olefinic double bonds. The above information,consistent with its molecular formula,reveals the molecule to have a tricyclic skeleton,which includes the skeleton of the oplopane sesquiterpene gmelinins A and B [17].
Spin systems of H-2/H-3/H-4,H-12/Me-13/Me-14 present in 2,as the analysis of the 1H-1H-COSY correlations revealed,which were assembled with the assistance of the HMBC correlations (Fig. 2). From the 1H-1H-COSY and HMBC correlations spectra,the partial structure of 2 contained a cyclopentane skeleton with an epoxide and an oxygen bridge adjacent to C-2 and C-3. The other two methyl groups attached at C-5 and C-6 were confirmed by the HMBC correlations from Me-10 to C-1,C-4 and C-6; Me-11 to C-5 and C-7. The HMBC correlations of H-2 to C-4,C-5,and C-8; H-3 to C-1 and C-5; H-4 to C-1,C-2,and C-6; H-7 to C-5,C-8,and C-12; and H-9 to C-2,C-5,and C-12; further supported the proposal structure for 2.
In the NOESY spectrum (Fig. 3),the correlations of H-2/H-3 and Me-10/H-4,revealed their β-orientations. Finally,the absolute configurations of C-1 and C-5,was determined by its CD spectrum,in which a positive Cotton effect by cyclohexene group was shown at 215 nm (Δε +6.2),whereas the reported value for ixerol B whose stereo-structures were determined by CD spectrum,was negative [18]. Although we do not have an explanation for the difference in the absolute values of the Cotton effect,appplicaton of the octant rule to the compound depicted in the formula of 2 found that the expected sign of the Cotton effect should be positive. These above observations suggested that 2 had the absolute configuration 1S,2R,3R,4S,and 5R. Thus,the structure of 2 was determined as showed in Fig. 1.
The acute toxicity investigation of these compounds was tested against brine shrimp A. salina and grouper E. akaara. The results are shown in Tables 2 and 3. The shrimp lethality results revealed that except compound 6,all the test compounds had significant brine shrimp lethality with LC50 values ranging from 2.2 to 13.7 μg/mL. The results of toxicity on E. akaara displayed that compounds 2,4,5,and 7 had moderate toxicity on E. akaara.
4. ConclusionIn conclusion,eight compounds,including two new compounds (1 and 2),were isolated from the P. globosa. The present study about the isolation and identification of eight compounds shows the diversity of chemical constituents in P. globosa. In addition,some of these compounds showed significant lethality on the brine shrimp A. salina and E. akaara larvae. The present study is the first report concerning natural products from wild P. globosa,which adds to the arsenal of antibiofouling chemicals.
5. AcknowledgmentsThis study was supported by grants from National Natural Science Foundation of China (No. 31100260,81260480,and 41566004),National Natural Science Foundation of Guangxi (No. 2011GXNSFE018002),Guangxi Key Laboratory of Marine Environmental Science,Guangxi Academy of Sciences (No. GXKLHY13-06),Foundation of Key Laboratory of Plant Resources Conservation and Sustainable Utilization,South China Botanical Garden,Chinese Academy of Sciences (No. 201210ZS).
| [1] | M.Y. Oh, S.B. Lee, D.H. Jin, Y.K. Hong, H.J. Jin, Isolation of algicidal compounds from the red alga Corallina pilulifera against red tide microalgae, J. Appl. Phycol. 22 (2010) 453-458. |
| [2] | B.Z. Zhang, G.J. Cai, H.T. Wang, et al., Streptomyces alboflavus RPS and its novel and high algicidal activity against harmful algal bloom species Phaeocystis globosa, PloS One 9 (2014) e92907. |
| [3] | L. Zhao, L.N. Chen, P.H. Yin, Algicidal metabolites produced by Bacillus sp. strain B1 against Phaeocystis globosa, J. Ind. Microbiol. Biotechnol. 41 (2014) 593-599. |
| [4] | X.W. Zheng, B.Z. Zhang, J.L. Zhang, et al., A marine algicidal actinomycete and its active substance against the harmful algal bloom species Phaeocystis globosa, Appl. Microbiol. Biotechnol. 97 (2013) 9207-9215. |
| [5] | T. Hase, Y. Kawamoto, K. Ohtani, et al., Cyclohexylethanoids and related glucosides from Millingtonia hortensis, Phytochemistry 39 (1995) 235-241. |
| [6] | W. Wang, W. Chen, Y.S. Yang, et al., New phenolic compounds from Coreopsis tinctoria Nutt. and their antioxidant and angiotensin I-converting enzyme inhibitory activities, J. Agric. Food Chem. 63 (2015) 200-207. |
| [7] | C.H. Kong, X.H. Xu, B. Zhou, et al., Two compounds from allelopathic rice accession and their inhibitory activity on weeds and fungal pathogens, Phytochemistry 65 (2004) 1123-1128. |
| [8] | J. Niebler, A. Buettner, Identification of odorants in frankincense (Boswellia sacra Flueck.) by aroma extract dilution analysis and two-dimensional gas chromatography-mass spectrometry/olfactometry, Phytochemistry 109 (2015) 66-75. |
| [9] | M.H. Hyun, S.C. Han, H.J. Choi, B.S. Kang, H.J. Ha, Effect of the residual silanol group protection on the liquid chromatographic resolution of racemic primary amino compounds on a chiral stationary phase based on optically active (3, 3'-diphenyl-1,1'-binaphthyl)-20-crown-6, J. Chromatogr. A 1138 (2007) 169-174. |
| [10] | H.H. Vogt, R. Gompper, The allopolarization principle and its applications, V. 13C NMR-spectra of enolates and related carbanions, Chem. Ber. Recl. 114 (1981) 2884-2897. |
| [11] | J.Y. Cho, Antifouling chromanols isolated from brown alga Sargassum horneri, J. Appl. Phycol. 25 (2013) 299-309. |
| [12] | B.N. Meyer, N.R. Ferrigni, J.E. Putnam, et al., Brine shrimp: a convenient general bioassay for active plant constituents, Planta Med. 45 (1982) 31-34. |
| [13] | X.M. Luo, S.H. Qi, H. Yin, C.H. Gao, S. Zhang, Alkaloids from the stem bark of Micromelum falcatum, Chem. Pharm. Bull. 57 (2009) 600-602. |
| [14] | C.H. Gao, L. Lin, B. Long, et al., A new diketopiperazine from the gorgonian coral Menella kanisa, Nat. Prod. Res. 28 (2014) 473-476. |
| [15] | Y.B. Cheng, C.Y. Chen, Y.H. Kuo, Y.C. Shen, New nitrogen-containing sesquiterpenoids from the Taiwanese soft coral Cespitularia taeniata May, Chem. Biodivers. 6 (2009) 1266-1272. |
| [16] | L. Yu, L. Lin, B. Long, et al., Menverins H-L, new highly oxygenated guaiane lactones from the gorgonian coral Menella kanisa, Helv. Chim. Acta 98 (2015) 710-718. |
| [17] | W.Z. Zeng, Q.Y. Quesheng, H. Zhang, Liang, Two new oplopane sesquiterpenes from Artemisia gmelinii Web. ex Stechm, Chin. Chem. Lett. 25 (2014) 1153-1156. |
| [18] | Y.F. Han, K. Gao, Z.J. Jia, Two new norsesquiterpenes from Ixeris polycephala, Chin. Chem. Lett. 17 (2006) 913-915. |
 2016, Vol.27
2016, Vol.27