In recent years,supercritical fluid extraction (SFE) has become an attractive alternative to conventional solvent extraction in many separation processes for the sake of both analytical and technical applications [1, 2, 3]. Analytical SFE mostly uses supercritical CO2 as an extractant because of low toxicity,relatively low costs and convenient critical properties (i.e. Tc = 31.1 ℃ and Pc = 7.38 MPa) [4]. In the past two decades,an increasing number of CO2 SFE studies that focus on metal ions have been published [5, 6, 7, 8]. Direct extraction of metal ions by supercritical CO2 is highly inefficient because of the charge neutralization requirement and weak solvent-solute interactions. One approach for metal ion extraction by supercritical CO2 is suggested to convert charged metal ions into neutral metal complexes by using a derivatization reagent in supercritical CO2 [9, 10]. Besides metal ion extraction,metal speciation studies have also been conducted using SFE with neat or modified CO2,including organotin [11, 12],organolead [13] and organomercury [10, 14]. However,there has been little research with a focus on arsenic speciation until now [15, 16].
Arsenic is widely distributed in the environment. Global arsenic pollution has become a serious problem with various adverse effects on human health [17, 18]. The most common arsenic species reported in environmental,clinical and other natural media include arsenite [As (Ⅲ)],arsenate [As (V)],monomethylarsonate (MMA) and dimethylarsinate (DMA) [19]. Unlike other toxic trace elements (e.g.,tin,lead,and mercury),the above inorganic arsenic species are more toxic than organic ones,and therefore an available analytical methodology that permits the quantitative extraction and detection of arsenic species in various solid matrices is needed. So far,several reviews about arsenic speciation techniques have been reported [20, 21, 22, 23]. Although high-performance liquid chromatography (HPLC) is the most commonly used separation technique for arsenic speciation,gas chromatography (GC) is often used after a prior derivatization step,which transforms original arsenic species into volatile and thermal stable derivatives such as trimethylsilyl derivatives [24],dithiocarbamate derivatives [25] and thioarsenite derivatives [26]. Among derivatization reagents,thioglycolic acid methyl ester (TGM) is used frequently. It does not only form thermally stable and lipophilic arsenic derivatives,but it can also convert alkylated organometallic forms MMA and DMA simultaneously with inorganic arsenic,as the sum of As (Ⅲ) plus As (V),into volatile sulfur derivatives [27, 28]. Therefore,it appeared possible to combine chemical derivatization-SFE with arsenic speciation in order to extract organic and inorganic arsenic species from solid matrices by SFE with on-matrix derivatization under supercritical conditions [29]. Compared with conventional sample extraction methods,this approach could reduce analysis time significantly by shortening the extraction period,eliminating the need for clean-up owing to extraction selectivity and providing the possibility of performing in situ derivatization reactions in fewer steps. Moreover,this procedure minimizes the usage of solvent and hazardous chemicals.
The main objective of the present study therefore was to develop a novel method in combination with in situ derivatization- SFE and GC for the speciation of MMA,DMA and inorganic arsenic in solid matrices using TGM as a derivatization reagent. As previous research has shown that TGM-derivatives might decompose under thermal stress at high temperatures,thioglycolic acid ethyl ester (TGE) was also evaluated as a derivatization reagent in order to avoid such degradation processes and get more stable arsenic derivatives [30]. The effects of pressure,temperature,flow rate of supercritical CO2,extraction time,modifier and microemulsion on extraction efficiency was studied. The procedure was also applied to the analysis of real soil and sediment samples.
2. Experimental2.1. Chemicals and standards
Standard solutions of As(Ⅲ) (1.011 mmol/L),As(V) (0.233 mmol/L),MMA (0.335 μmol/mL) and DMA (0.706 μmol/ mL) were supplied by the China Standard Certification Center (CSC) and stored at 4 ℃ in PTFE bottles. Ultrapure water (18 MΩ),obtained by using a Milli-Q water purification system (Millipore,USA),was used throughout. All glassware was cleaned by using 10% (v/v) nitric acid (Merck KGaA,Germany),followed by multiple rinses with ultrapure water.
The supercritical grade carbon dioxide (99.99%) was supplied by Jinan Gas Factory of Shandong Province in China. TGM and TGE were purchased from Shanghai Chemical Reagent Company of the Medicine Group of China. Cyclohexane (Chemical Experimental Factory of Tianjin University,China) was used as the collecting solvent. HPLC grade methanol (TEDIA Co.,USA) was used as modifier solvent. Triton X-100 (Beijing Reagent Factory,China) was used to form microemulsion,and silica gel (Ocean Chemical Factory of Qingdao,China) was used as the solid matrix. All other chemicals used were analytical grade reagents.
Nitrogen (99.99%,Jinan Gas Factory,China) was used as the carrier gas for GC. Tetradecane (Shanghai Chemical Reagent Co.,Ltd.,China) was added as the internal standard (IS) for chromatography in a 100 mg/L solution in cyclohexane.
The certified reference material soil GSS-1 and sediment GSD- 10 were purchased from the Institute of Geophysical and Geochemical Exploration (IGGE) of China.
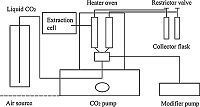
2.2. InstrumentationSFE: The extraction experiments were performed using a SFE system (Applied Separation Co.,USA),the schematic diagram of which is shown in Fig. 1. Two 5 mL stainless-steel extraction vessels were fitted with stainless-steel needle valves,which could be applied for two samples simultaneously. Two micrometering valves were used as restrictor valves to control the flow rate of the supercritical CO2 to the solvent collection. The modifier (methanol) was added by an HPLC pump (WellChrom K-501,Germany) to the supercritical CO2 stream. The extraction temperatures were monitored by a thermocouple with precision of ± 1 ℃.
|
Download:
|
| Fig. 1.The scheme diagram of the SFE system. | |
GC: The arsenic derivatives were determined by a GC-2014 (Shimadzu,Japan) gas chromatograph equipped with a flame ionization detector (FID). The Shimadzu GC Solution Chromatography Data System was used to control and automate the GC systems,and for data acquisition and analysis. The column was a 30 m × 0.25 mmi.d. Restek Rtx-5 capillary column with a 0.25 μm film thickness (crossbond 5% diphenyl -95% dimethylpolysiloxane,Restek Co.,USA). Nitrogen was used as the carrier gas at a flow rate of 3.0 mL/min. The temperature program adopted was as follows: set at 50 ℃ and held for 0.5 min initially,then increased up to 230 ℃ at 20 ℃/min and then to 300 ℃ at 40 ℃/min,where it was held for 1 min. Sample volumes of 2 μL were injected into a liner of 900 μL internal volume,in the splitless injection mode with an injection port temperature of 240 ℃. The temperature of the detector was set at 300 ℃.
2.3. ProcedureAn aqueous arsenic solution was prepared by dilution from the standard solutions in ultrapure water and stored at 4 ℃ for not more than one week. The simulated sample was prepared as follows. A known amount of mixed arsenic solutions was spiked onto silica gel and dried at 105 ℃. The dry sample was mixed well again. Concentrations were 5 mg/kg sample [As(Ⅲ) and As(V)] and 1 mg/kg (MMA and DMA).
One of the end-fittings was removed and a plug of polypropylene wool was pushed into the closed end of the SFE vessel. The 5 mL vessel was filled with 2.0 g of the prepared simulated sample. The sample bed was packed firmly to ensure the uniform diffusion of supercritical CO2 throughout the sample matrix. An aliquot (200 μL) of TGM or TGE was added and the remainder of the cell volume was also filled with polypropylene wool. Then the vessel was installed into the oven to perform the extraction. As the modifier solvent,the methanol solution was added into the extraction cell by the HPLC pump.
In order to find the optimum extraction conditions for arsenic derivatives,different pressures,temperatures,extraction time,flow rates of supercritical CO2 and methanol modifier proportion were studied. Under the optimum conditions,a 1 mL aliquot of microemulsion,containing Triton X-100 (13.3% + cyclohexane 73.3% + 1-butanol 13.3%,w/w),was added to the extraction cell to study the effect of the surfactant on the extraction efficiency. The component and ratio of microemulsion coincided with the results found in previous studies [9, 31]. The arsenic derivatives were collected in 2 mL of cyclohexane,and 2 μL of the liquid was injected and analyzed by GC as described in Section 2.2.
For reference,an aqueous solution that contained the same amount of arsenic species as the simulated sample was derivatized by TGM or TGE and analyzed by GC. The procedure was conducted as follows. The aqueous samples (5 mL) with known amounts of As(Ⅲ),As(V),MMA and DMA were placed in 10 mL capped glass tubes. Subsequently 50 μL 1 mol/L HCl and 150 μL derivatization reagents were added. The tubes were closed and shaken intensively for 2 min at ambient temperature. After adding 2 μL of cyclohexane,shaking was continued for another two minutes. Finally,2 mL of the upper organic phase of the reaction mixture was injected into the GC injection port. The parameters used above have been optimized in preliminary experiments and more than 99% of arsenic species in aqueous samples can be extracted and determined. The recoveries of arsenic species were calculated by comparing the chromatographic result obtained by using the SFE technique with the result obtained by using the derivatization method in aqueous solution.
The extraction efficiencies of arsenic species from the standard reference soil sample (GSS-1) and sediment sample (GSD-10) were determined under the optimum extraction conditions. Recovery experiments were carried out using two reference samples as well as real soil and sediment samples spiked with the arsenic species at three concentration levels,ranging from 5 to 50 mg/kg for inorganic arsenic and from 0.5 to 5 mg/kg for DMA and MMA. The spiked method was performed in the same manner as the simulated samples. Triplicate determinations were made in each experiment to ensure reproducibility.
3. Results and discussionThe derivatization reactions of As(Ⅲ),As(V),MMA and DMA using TGM were developed by Beckerman [27] and Dix [19]. Referring to these approaches and previous studies,TGE was also evaluated as a derivatization reagent with similar reaction pathways [28]. The same derivatives were formed from pentavalent and trivalent arsenic due to the high reducing power of TGM and TGE. The derivatization reaction indicated that DMA,MMAand inorganic arsenic were substituted by one,two and three thioglycol side-chains respectively,which could be extracted by supercritical CO2 and separated by GC for their different polarities and volatility.
The selection of experimental conditions for SFE was influenced by several factors including pressure,temperature,flow rate of supercritical CO2,extraction time and modifier. Suitable amounts of modifier have a large impact on the extraction efficiencies of the arsenic derivatives. In this paper,methanol was used as the modifier for supercritical CO2 to reduce extraction time and optimize extraction conditions.
3.1. Effect of pressure on recoveries of arsenic speciesThe recoveries of arsenic species under different pressures of the methanol-modified supercritical CO2 are shown in Table S1 in Supporting information. It was observed that the recoveries of arsenic species using two derivatization reagents showed similar characteristics,i.e. rose increasingly with the rise of pressure from 10 MPa to 30 MPa and then decreased gradually with continued rise of pressure. The fluid pressure is an essential parameter in SFE because the fluid density is directly related to pressure [6]. With the increase of supercritical CO2 pressure,the density and solvation power of the supercritical CO2 increased correspondingly,leading to an increase of solubility of arsenic derivatives in supercritical CO2,which was beneficial to the extraction. However,when the pressure reached a threshold value,the increased pressure retarded the mass transfer rate of arsenic derivatives extraction from the matrices. In addition,the loss of arsenic derivatives increased with the increase of pressure due to both volatilization and the formation of aerosols [9, 16]. In this study,a value of 30 MPa was selected as the optimum pressure.
3.2. Effect of temperature on recoveries of arsenic speciesThe effects of temperature on the recoveries of arsenic species with the methanol-modified supercritical CO2 are shown in Table S2 in Supporting information. The results showed that extraction efficiencies ofMMA and DMA increased constantly with the rise of the temperature from 40 ℃ to 100 ℃ and then decreased,while inorganic arsenic had an extraction efficiency that increased constantly with the rise of the temperature. According to the rule of kinetics,the higher the temperature,the more intensive the heat motion of solutes on active sites of the matrix,which is helpful for solutes to overcome the adsorbing energy fortress of the matrix and desorb more efficiently from active sites of the matrix by supercritical CO2. In view of thermodynamics,the saturated vapor pressure and solubility increased correspondingly with the increase of temperature,making the arsenic derivatives dissolve in supercritical CO2 more easily [31]. Moreover,when temperature increased,the density of supercritical CO2 decreased,which made the solubilizing ability of supercritical CO2 decrease; therefore,the solubility of arsenic derivatives in supercritical CO2 decreased [32]. These three kinds of functions competed with each other. In comprehensive consideration of simultaneous extraction of DMA,MMA and inorganic arsenic,this study selected 100 ℃ as the optimum extraction temperature.
3.3. Effect of the modifier and surfactant on recoveries of arsenicspecies Improved solvent characteristics and extraction efficiency could be obtained by adding a polar solvent such as methanol to supercritical CO2. The role of the polar modifier was probably to cover adsorption sites of the matrix,so as to interrupt strong analyte-matrix interactions and promote rapid desorption of arsenic derivatives into the supercritical fluid or prevent readsorption. In addition,modifier can increase the solubilizing ability of supercritical CO2,so that efficient elution condition could be maintained [9]. Methanol was commonly chosen as the modifier because of its high polarity and its ability to deactivate active sites on the surface of solid matrix. This study investigated the percentage (2-10%,v/v) of methanol in supercritical CO2. As can be seen in Table S3 in Supporting information,TGM and TGE had similar effects as derivatization reagents,i.e. the recoveries of arsenic species can be substantially improved by adding a certain amount of methanol modifier compared with pure supercritical CO2. The result suggested that the concentration of modifier in supercritical CO2 had little effect on the extraction efficiency of the arsenic derivatives. As indicated in the research done by Langenfeld et al.,the modifier concentration was based on the volume of the empty extraction cell and was altered slightly by the presence of the sample [33]. This study selected 5% (v/v) methanol as the modifier concentration.
According to previous research,the extraction efficiency of many types of metal derivatives by methanol modified supercritical CO2 can be further improved by the presence of a microemulsion containing Triton X-100 [31, 34]. Surfactant Triton X-100,nonpolar solvent cyclohexane and polar metal derivatives could be stable in thermodynamics,isotropism and transparent disperse system by using 1-butanol and other straight-chain alcohols with medium-length carbon chain [9]. This study indicates that the extraction efficiency of DMA,MMA and inorganic arsenic derivatives could also be promoted further by adding a microemulsion containing Triton X-100. The reason for this is probably that the microemulsion can lower the analyte polarity based on reverse micelle formation,which was beneficial to the desorption of arsenic derivatives from active sites of matrix to supercritical CO2 and the dissolution of arsenic derivatives in supercritical CO2 [35, 36]. Thus,this study added a microemulsion containing Triton X-100 before putting the sample into an SFE vessel,in order to improve the extraction efficiency of arsenic species.

|
Download:
|
| Fig. 2.GC chromatograms of: (A) soil references material (GSS-1); (B) GSS-1 spiked at the concentration levels of 5, 5 and 50 mg/kg for DMA, MMA and inorganic arsenic, respectively. TGE was used as derivatization reagent. Peaks: (1) DMA derivative; (2) TGE disulfide; (3) MMA derivative; (4) inorganic derivative. | |
the recoveries of arsenic species The effects of the dynamic extraction time on the recoveries of arsenic species are shown in Table S4 in Supporting information. The static extraction time was set at 10 min according to preliminary experiments. The results indicated that when the dynamic extraction time of SFE increased,extraction efficiencies improved constantly. In research that applied the SFE approach,static extraction and dynamic extraction were often combined. In the static method,the long exposure to solvent allows the matrix to swell,thus improving the penetration of supercritical CO2 into the interstices of matrix and increasing the recoveries of arsenic derivatives,while in the dynamic method,continual exposure of the arsenic derivatives to fresh solvents enhances partitioning of the derivatives into supercritical CO2. In this study,once the arsenic derivatives outflow from the outlet valve,more than 96% of them can be completely extracted within 25 min. The efficiency data remained almost the same in the range of 25-40 min,and 25 min was selected as the optimum dynamic extraction time.
Furthermore,the effects of the flow rate of supercritical CO2 (0.5-4.0 mL/min) on the recoveries of arsenic species were studied. It was observed that with the increase of the flow rate,the recoveries of arsenic derivatives using the two kinds of derivatization reagents increased and a shorter amount of time was required to get the same extraction efficiency. This is because that higher flow rate of supercritical CO2 could enhance the mass transfer coefficient and increase the extraction ability of supercritical CO2. Thus,the recoveries of the arsenic derivatives were elevated. However,with the further increase of the flow rate of supercritical CO2,extraction efficiencies decreased due to aerosol formation. So the flow rate of supercritical CO2 was controlled at 1.0 mL/min in this study.
3.5. Gas chromatographyGC is widely used for the separation and quantification of TGM derivatives of arsenic [20, 21]. Referring to these approaches,the reaction of target analytes with TGE was also evaluated. The three arsenic species could be separated from simulated solid samples and detected in the lower mg/kg range. Fig. 2(B) shows a gas chromatogram of the spiked reference soil sample (GSS-1) using TGE as the derivatization reagent,showing that retention times of DMA,MMA and inorganic arsenic are 5.78 min,10.1 min and 11.4 min,respectively. Three kinds of arsenic derivatives using TGM as the derivatization reagent had similar peak characteristics; DMA,MMA and inorganic arsenic had retention time of 5.5 min,9.8 min and 10.4 min,respectively. The peak order was consistent with previous studies [19, 37, 38].
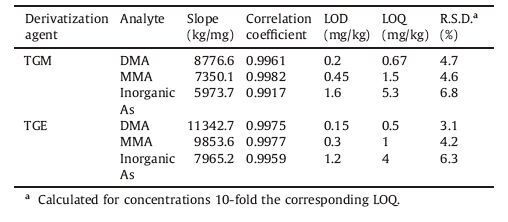
The calibration was performed for DMA,MMA and inorganic arsenic using the optimized procedure to analyze simulated solid matrices. All analytical parameters are summarized in Table 1. Seven concentration levels were analyzed and two replicates were made. The method was selective and a linear correlation was obtained at concentration levels ranging from 2 to 100 mg/kg for three arsenic species. The correlation coefficients obtained demonstrated a direct proportional relationship between the amount of analyte extracted and its concentration in the simulated sample. By comparing slopes of the linear regression,the detection sensitivity of three arsenic species can be ordered as DMA > MMA > inorganic arsenic. Reproducibility measurements showed an acceptable relative standard deviation (R.S.D.) in the range of 1.8%-5.5% for DMA,1.5%-5.9% for MMA and 2.6%-7.7% for inorganic arsenic.
|
|
Table 1 Analytical characteristics of DMA, MMA and inorganic arsenic using SFE–GC method for simulated solid matrices (n = 6). |
The analytical results for both derivatization procedures were compared concerning the sensitivity to find the most efficient derivatization reagent. This study showed that arsenic species that react with TGE had larger peak areas under the same parameters,reflecting a better sensitivity and therefore a lower limit of detection for the quantification of arsenic species. Richter et al. compared peak characteristics of TGM and TGE as derivatization reagents for speciation of MMA and DMA by the GC-MS method,and similar results were obtained [28]. In this study,the limits of detection (LOD) and limits of quantification (LOQ) were calculated by using a signal-to-noise ratio of three and ten,respectively. As shown in Table 1,when TGM was used as a derivatization reagent,the LOD were found to be 0.2,0.45 and 1.6 mg/kg for DMA,MMA and inorganic arsenic,respectively. However,the LOD were found to be 0.15,0.3 and 1.2 mg/kg for DMA,MMA and inorganic arsenic when TGE was used as a derivatization reagent,showing a lower detection limit for three arsenic species. Thus TGE was used as a derivatization reagent for the analysis of real solid samples and recovery experiments.
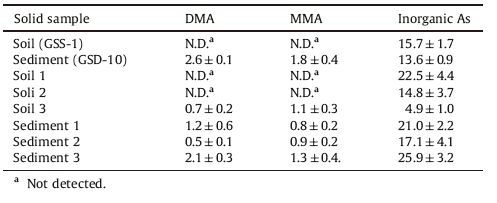
3.6. Analysis of extracted real solid samples and validation of the methodThe optimized SFE-GC procedure using TGE as a derivatization reagent was applied to the standard reference soil sample (GSS-1) and sediment sample (GSD-10). In addition,three real soil samples and three real sediment samples were also analyzed. Soil samples were collected from urban surface soil (soil 1),urban industrial soil (soil 2) and suburban farmland soil (soil 3) respectively; while sediment samples were collected from the Yellow River (sediment 1),the Daming Lake (sediment 2) and a reservoir (sediment 3),respectively. Real solid samples were dried at 105 ℃,grinded and screened by a 0.096 mm mesh. The results are shown in Table 2. Inorganic arsenic was detected in all real solid samples and exhibited higher content thanMMAand DMA. The two organic arsenic species were not detected in soil samples except soil 3,indicating that the main arsenic species in soil samples were inorganic arsenic. However,organic arsenic was detected in soil 3,which can be attributed to the agricultural fertilizing activities of this region and geologic features of the soil collection area [39]. Organic arsenic could be detected in all sediment samples,indicating that an aqueous environment was more conducive to the formation or storage of organic arsenic.
|
|
Table 2 Extraction of arsenic species from real solid samples (mg/kg; n = 3). |
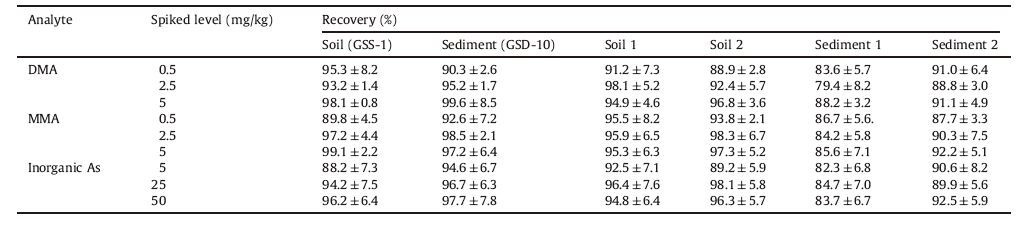
The reliability of the analysis procedure was determined by recovery experiments,in which TGE was used as the derivatization reagent for six different solid samples spiked at three concentration levels,as can be seen in Table 3. GC chromatograms of soil reference material (GSS-1) and spiked GSS-1 are shown in Fig. 2. The average recoveries ± standard deviations of DMA,MMA and inorganic arsenic were 92.0 ± 4.7,93.2 ± 5.3,92.1 ± 6.7,respectively. Slight differences were observed between the R.S.D. values obtained for inorganic arsenic in the spiked samples and the values obtained for the organic arsenic compounds.
|
|
Table 3 Mean recovery efficiencies obtained in spiked samples by using the SFE–GC method (n = 3). |
In conclusion,a method in combination with in situ derivatization- SFE and GC for the speciation and quantitative determination of DMA,MMA and inorganic arsenic simultaneously in solid matrices was evaluated for the first time. The results of the study showed that TGE is a more effective derivatization reagent than TGM. Necessary conditions for efficient extraction were systematically researched. The use of supercritical CO2 with a suitable amount of methanol as a modifier produced higher recoveries of arsenic species compared with those obtained by pure supercritical CO2. Significant enhancement of extraction efficiencies were observed in the presence of a microemulsion containing Triton X- 100. Agreeable values for recovery and precision were obtained. The resulting method was fast,easy to perform and selective in the extraction and detection of various arsenic species in solid matrices,and could minimize the usage of solvent and hazardous chemicals. The proposed analytical method shows its potential as a remediation technique for arsenic pollution in soil and other solid samples.
5. AcknowledgmentThis work was financially supported by Environmental Protection and Public Welfare Industry Research Special: the remediation technologies and demonstration for the combined pollution of the oil-heavy metals in the saline soil (No. 201109022). The authors are also grateful to the support by National High-tech Research and Development Projects (National 863 Projects): the key technology of efficient exploiting deep brine in the Yellow River delta (No. 2012AA061705).
6. Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.10. 001.
| [1] | K. Park, J. Lee, J. Sung, Metal extraction from the artificially contaminated soil using supercritical CO2 with mixed ligands, Chemosphere 91 (2013) 616-622. |
| [2] | D.L. Quach, B.J. Mincher, C.M. Wai, Supercritical fluid extraction and separation of uranium from other actinides, J. Hazard. Mater. 274 (2014) 360-366. |
| [3] | J. Sunarso, S. Ismadji, Decontamination of hazardous substances from solid matrices and liquids using supercritical fluids extraction: a review, J. Hazard. Mater. 161 (2009) 1-20. |
| [4] | S.B. Hawthorne, Analytical-scale supercritical fluid extraction, Anal. Chem. 62 (1990) 633-642. |
| [5] | K. Sujatha, K.C. Pitchaiah, N. Sivaraman, et al., Recovery of plutonium from polymeric waste matrices using supercritical fluid extraction, Desalin. Water Treat. 52 (2014) 470-475. |
| [6] | C.M. Wai, S.F. Wang, Supercritical fluid extraction: metals as complexes, J. Chromatogr. A 785 (1997) 369-383. |
| [7] | C.M. Wai, S.F. Wang, Separation of metal chelates and organometallic compounds by SFC and SFE/GC, J. Biochem. Biophys. Methods 43 (2000) 273-293. |
| [8] | Y.H. Lin, N.G. Smart, C.M. Wai, Supercritical fluid extraction and chromatography of metal chelates and organometallic compounds, TrAC, Trends Anal. Chem. 14 (1995) 123-133. |
| [9] | J.C. Liu, W. Wang, G.Z. Li, A new strategy for supercritical fluid extraction of copper ions, Talanta 53 (2001) 1149-1154. |
| [10] | Y. Liu, V. Lopez-Avila, M. Alcaraz, W.F. Beckert, E.M. Heithmar, Determination of metals in solid samples by complexation-supercritical fluid extraction and gas chromatography-atomic emission detection, J. Chromatogr. Sci. 31 (1993) 310-316. |
| [11] | Y.K. Chau, F. Yang, M. Brown, Supercritical fluid extraction of butyltin compounds from sediment, Anal. Chim. Acta 304 (1995) 85-89. |
| [12] | V. Lopez-Avila, Y. Liu, W.F. Beckert, Interlaboratory evaluation of an off-line supercritical fluid extraction and gas chromatography with atomic emission detection method for the determination of organotin compounds in soil and sediments, J. Chromatogr. A 785 (1997) 279-288. |
| [13] | P. Quevauviller, L. Ebdon, R.M. Harrison, Y. Wang, Certification of trimethyl-lead in an urban dust reference material (CRM 605), Appl. Organomet. Chem. 13 (1999) 1-7. |
| [14] | H. Emteborg, E. Björklund, F. Ödman, et al., Determination of methylmercury in sediments using supercritical fluid extraction and gas chromatography coupled with microwave-induced plasma atomic emission spectrometry, Analyst 121 (1996) 19-29. |
| [15] | J.M. Bayona, Supercritical fluid extraction in speciation studies, TrAC, Trends Anal. Chem. 19 (2000) 107-112. |
| [16] | M. Ashraf-Khorassani, M.T. Combs, L.T. Taylor, Supercritical fluid extraction of metal ions and metal chelates from different environments, J. Chromatogr. A 774 (1997) 37-49. |
| [17] | S. Kapaj, H. Peterson, K. Liber, P. Bhattacharya, Human health effects from chronic arsenic poisoning—a review, J. Environ. Sci. Health., A: Toxic Hazard. Subst. Environ. Eng. 41 (2006) 2399-2428. |
| [18] | J.C. Ng, J.P. Wang, A. Shraim, A global health problem caused by arsenic from natural sources, Chemosphere 52 (2003) 1353-1359. |
| [19] | K. Dix, C.J. Cappon, T.Y. Toribara, Arsenic speciation by capillary gas-liquid chromatography, J. Chromatogr. Sci. 25 (1987) 164-169. |
| [20] | I. Ali, C.K. Jain, Advances in arsenic speciation techniques, Int. J. Environ. Anal. Chem. 84 (2004) 947-964. |
| [21] | M.L. Chen, L.Y. Ma, X.W. Chen, New procedures for arsenic speciation: a review, Talanta 125 (2014) 78-86. |
| [22] | B. Radke, L. Jewell, J. Namieśnik, Analysis of arsenic species in environmental samples, Crit. Rev. Anal. Chem. 42 (2012) 162-183. |
| [23] | Y.G. Yin, J.F. Liu, G.B. Jiang, Recent advances in speciation analysis of mercury, arsenic and selenium, Chin. Sci. Bull. 58 (2013) 150-161. |
| [24] | F.T. Henry, T.M. Thorpe, Gas chromatography of the trimethylsilyl derivatives of arsenic, arsenious, and dimethylarsinic acids, J. Chromatogr. A 166 (1978) 577-586. |
| [25] | E.H. Daughtrey Jr., A.W. Fitchett, P. Mushak, Quantitative measurements of inorganic and methyl arsenicals by gas-liquid chromatography, Anal. Chim. Acta 79 (1975) 199-206. |
| [26] | D.R. Killelea, J.H. Aldstadt Ⅲ, Solid-phase microextraction method for gas chromatography with mass spectrometric and pulsed flame photometric detection: studies of organoarsenical speciation, J. Chromatogr. A 918 (2001) 169-175. |
| [27] | B. Beckermann, Determination of monomethylarsonic acid and dimethylarsinic acid by derivatization with thioglycolic acid methylester and gas-liquid chromatographic, Anal. Chim. Acta 135 (1982) 77-84. |
| [28] | J. Richter, S. Lischka, C. Piechotta, Analysis of arsenic species in fish after derivatization by GC-MS, Talanta 101 (2012) 524-529. |
| [29] | B.W. Wenclawiak, M. Krah, Reactive supercritical fluid extraction and chromatography of arsenic species, Fresenius J. Anal. Chem. 351 (1995) 134-138. |
| [30] | K. Schoene, J. Steinhanses, H.J. Bruckert, A. König, Speciation of arsenic-containing chemical warfare agents by gas chromatographic analysis after derivatization with thioglycolic acid methyl ester, J. Chromatogr. A 605 (1992) 257-262. |
| [31] | Z.J. Cui, G.Y. Zhang, W. Song, Y.T. Song, Supercritical fluid extraction of metal ions from a solid matrix with 8-hydroxyquinoline and carbon dioxide, J. Liq. Chromatogr. Related Technol. 27 (2004) 985-994. |
| [32] | Y. Yamini, M. Asghari-Khiavi, N. Bahramifar, Effects of different parameters on supercritical fluid extraction of steroid drugs, from spiked matrices and tablets, Talanta 58 (2002) 1003-1010. |
| [33] | J.J. Langenfeld, S.B. Hawthorne, D.J. Miller, J. Pawliszyn, Role of modifiers for analytical-scale supercritical fluid extraction of environmental samples, Anal. Chem. 66 (1994) 909-916. |
| [34] | S.Y. Du, G.Y. Zhang, Z.J. Cui, Supercritical fluid extraction of hazardous metals from urban total suspended particles, J. Liq. Chromatogr. Related Technol. 28 (2005) 1487-1495. |
| [35] | M.M. Jiménez-Carmona, M.D. Luque de Castro, Reverse micelle formation for acceleration of the supercritical fluid extraction of cholesterol from food samples, Anal. Chem. 70 (1998) 2100-2103. |
| [36] | M.M. Jiménez-Carmona, M.D. Luque de Castro, Reverse-micelle formation: a strategy for enhancing CO2-supercritical fluid extraction of polar analytes, Anal. Chim. Acta 358 (1998) 1-4. |
| [37] | F.A. Claussen, Arsenic speciation of aqueous environmental samples by derivatization with thioglycolic acid methylester and capillary gas-liquid chromatography-mass spectrometry, J. Chromatogr. Sci. 35 (1997) 568-572. |
| [38] | Z. Mester, J. Pawliszyn, Speciation of dimethylarsinic acid and monomethylarsonic acid by solid-phase microextraction-gas chromatography-ion trap mass spectrometry, J. Chromatogr. A 873 (2000) 129-135. |
| [39] | A.J. Bednar, J.R. Garbarino, J.F. Ranville, T.R. Wildeman, Presence of organoarsenicals used in cotton production in agricultural water and soil of the southern united states, J. Agric. Food Chem. 50 (2002) 7340-7344. |
 2016, Vol.27
2016, Vol.27