Chloramphenicol (CAP),as a broad-spectrum antibiotic,has a strong bactericidal power and therapeutic efficacy [1]. However,it has serious toxic side effects on hematopoietic system such as aplastic anemia [2]. In addition,long-term intake of a trace of CAP can cause the imbalance of body’s normal flora. To build highly sensitive detection of CAP therefore has important significance. So far,many methods have been used for the determination of CAP,such as gas chromatography-mass spectrometry [3, 4],high performance liquid chromatography [5],capillary zone electrophoresis [6],chemiluminescence [7],electrochemical sensor [8] etc. Among these methods,electrochemical sensor possesses the advantages of low cost,rapid analysis speed and high sensitivity.
As an outstanding conducting polymer with excellent electrochemical properties,biocompatibility and chemical stability,polyaniline (PANI) has been a promising material in sensing fields [9]. Moreover,in order to improve the performances and applications of PANI-based sensors,PANI was often combined with other functional materials. For example,Yang et al. prepared an ERGNO/PANI-based DNA sensor which showed a wide linear range and low detection limit due to the synergistic effect of ERGNO and PANI [10].
MoS2 is a type of layered transitionmetal sulfide that constructed of three atom layers stacked by weak van der Waals forces and possesses an analogous structure to graphene [11]. It is commonly used as a solid lubricant aswell as catalysts for hydrodesulfurization reaction and hydrogen evolution reaction [12, 13]. In recent years,due to its layered structure,MoS2 has attracted great attention in the fields of electrochemistry and electronics. Integrating MoS2 with other functional materials,can bring improved properties due to their synergistic effect. For example,MoS2/graphene composites exhibited more excellent electrochemical performances than MoS2 or graphene in lithium ion battery [14]. Although MoS2/PANI have been synthesized and used in lithium ion batteries [15, 16],the application of MoS2/PANI nanocomposite in electrochemical sensing has been reported scarcely.
In this work,the thin-layered MoS2/PANI nanocomposite was synthesized via the combination of ultrasonic exfoliation of bulk MoS2 and in situ polymerization of aniline. The as-prepared MoS2/ PANI nanocomposite exhibited excellent conductivity and large electroactive surface area. Furthermore,we developed a new sensitive electrochemical sensor based on this nanocomposite for the detection of CAP. Due to the special 3D structure of MoS2/PANI nanocomposite easily absorbing the conjugated CAP and the excellent electrochemical performance,the sensor achieved the highly sensitive detection of CAP with a wide detection range and low detection limit.
2. Experimental2.1. Apparatus and reagents
Electrochemical measurements were performed on a CHI 760D electrochemical workstation (Shanghai CH Instrument Company,China) with a three-electrode system. A saturated calomel electrode and a platinum wire were used as reference electrode and counter electrode respectively,and a bare carbon paste electrode (CPE) or modified CPE was used as working electrode. The ultrasonic process was performed with a KQ-500B sonifier. The morphology of as-prepared composite was characterized by JSM- 6700F scanning electron microscopy (SEM),JEM-2100 transmission electron microscopy (TEM). X-ray diffraction (XRD) measurement was investigated on a Rigaku D/Max-2=0 diffractometer with Cu Ka radiation.
Bulk MoS2,aniline,ammonium persulfate and CAP were respectively purchased from BASF Chemical Co.,Ltd. (Tianjin,China),Tianjin Damao Chemical Factory,Tianjin Guangcheng Chemical Co.,Ltd. and Shanghai Bioengineering Co.,Ltd. The CAP stock solution was prepared with ethanol and then diluted to the given concentration using 0.3 mol/L phosphate buffer solution (PBS). All other reagents were of analytical grade,commercially available and used as received. Aqueous solutions were made with ultrapure water.
2.2. Preparation of MoS2/PANI nanocompositeA certain amount of MoS2 was dispersed in 80 mL of 1 mol/L HClO4 aqueous solution and sonicated for 4 h to get thin-layered MoS2 dispersion. Then 0.465 g of aniline and 10 mL of ethanol were added. Afterward,the obtained mixture was stirred and put in refrigerator at -10 ℃. At the same time,0.76 g of ammonium persulfate was dissolved in 10 mL of 1 mol/L HClO4 aqueous solution,and then the resulting solution was put in refrigerator at -10 ℃ as well. 20 min later,the two dispersions were mixed,and the obtained mixture reacted at -10 ℃ for 18 h. Finally,a precipitate was collected by filtration and washed with ultrapure water until the filtrate became clear,then naturally dried at room temperature. The resulting lump solid was grounded at room temperature to give a black powder. The MoS2/PANI nanocomposite was obtained.
In addition,pure PANI was prepared using similar method but in absence of MoS2.
2.3. Fabrication of the modified electrodesThe carbon paste electrode (CPE) was prepared by Yang et al.’s report [17]. 0.5 g/L MoS2/PANI made with ultrapure water was sonicated for 1 h,20 μL of which was dripped onto the tip of CPE and naturally dried in the air at room temperature. The obtained electrode was denoted as MoS2/PANI/CPE. Similarly,PANI/CPE was prepared. And 0.5 g/L thin-layered MoS2 suspension was obtained from dispersing bulk MoS2 in DMF and then sonicating it for 4 h. Then MoS2/CPE was obtained in the same way.
2.4. Electrochemical measurementsDifferential pulse voltammetry (DPV) measurements were carried out in 0.3 mol/L PBS supporting electrolyte. The scan range was from -0.3 V to -1.2 V,and the pulse amplitude,pulse width and pulse period were 0.05 V,0.06 s and 0.2 s,respectively.
In this paper,the reported result was the average of three parallel measurements.
3. Results and discussion3.1. Characterization of the MoS2/PANI nanocomposite
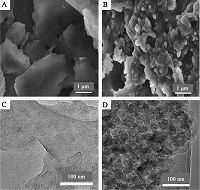
The representative SEM and TEM images of MoS2 and MoS2/ PANI are shown in Fig. 1. From Fig. 1A,we can see the thin-layered structure of MoS2,and its surface is comparatively smooth. The thin-layered structure can be further confirmed by the TEM image (Fig. 1C). Fig. 1B shows that lots of amorphous nanoparticles appeared on the surfaces and interlayer of the thin-layered MoS2 to form a 3D structure. Furthermore,the TEM image (Fig. 1D) displays many dark shadows which belong to the PANI nanoparticles. It indicates that the thin-layered MoS2 has successfully integrated with the PANI,which was further confirmed by the XRD characterization of MoS2/PANI nanocomposite (see Supporting information Fig. S1). Moreover,among these materials,the MoS2/ PANI nanocomposite has the highest conductivity and largest electroactive surface area (Fig. S2,Table S1 in Supporting information).
|
Download:
|
| Fig. 1.SEM images of (A) MoS2 and (B) MoS2/PANI and TEM images of (C) MoS2 and (D) MoS2/PANI. | |
In order to get the optimal composite,different quantity of MoS2 (0.009 g,0.018 g,0.036 g) were used to prepare series of MoS2/PANI nanocomposites and the corresponding modified electrodes were denoted as MoS2 (0.009 g)/PANI/CPE,MoS2 (0.018 g)/PANI/CPE and MoS2 (0.036 g)/PANI/CPE. The DPV measurements of 1.0 × 10-4 mol/L CAP at different electrodes were investigated. From Fig. 2,we can see different modified electrodes have different response values. Compared curves b and c with a,it can be known both PANI and MoS2 have catalytic effect for electrochemical reduction of CAP. But the catalytic effect of MoS2 is relatively poor,which is maybe due to the low conductivity of MoS2. However,MoS2/PANI/CPEs (curves d,e and f) display the larger reduction peak currents as compared with MoS2/CPE (curve b) and PANI/CPE (curve c). It indicates that MoS2/PANI get a synergistic catalytic effect for CAP,which may be due to the physisorption interaction between aromatic PANI and the basal plane of MoS2 and the excellent electrochemical performance of MoS2/PANI nanocomposite. Besides,the special structure of MoS2/ PANI nanocomposite can easily absorb the conjugated CAP,as a result,it could facilitate electron exchange between the CAP and the electrode surface and further provide an excellent platform for CAP detection.

|
Download:
|
| Fig. 2.DPVs of 1.0 × 10-4 mol/L CAP at (a) CPE, (b) MoS2/CPE, (c) PANI/CPE, (d) MoS2 (0.009 g)/PANI/CPE, (e) MoS2 (0.018 g)/PANI/CPE and (f) MoS2 (0.036 g)/PANI/CPE. | |
Furthermore,with increasing the content of MoS2 in MoS2/PANI nanocomposite,the current response increases at first but then decreases,and the MoS2 (0.018 g)/PANI/CPE displays the maximal peak current,which might be due to the largest electroactive surface area and highest conductivity. Therefore,the MoS2 (0.018 g)/PANI/CPE is the optimization.
The influence of pH for the electrochemical behavior of CAP at MoS2/PANI/CPE was studied,and the optimal pH value is 7.0 (see Supporting information Fig. S3).
3.3. Detection of CAP with a series of concentrationsUnder the optimum conditions,we investigated the CAP with a series of concentrations,and the results are displayed in Fig. 3. It is apparent that with the increase of the concentration of CAP,the peak current increases as well (Fig. 3A). As is depicted in Fig. 3B,the relationship between peak current values (Ip) and the concentrations (C) of CAP was described as two line segments. In the low concentrations range (0.1-10 μmol/L) the linear relationship can be denoted as Ip (μA) = 0.7656C (μmol/L) + 1.7042 (R2 = 0.9925) while in the high concentrations range (10-100 μmol/L) it can be denoted as Ip (μA) = 0.2404C (μmol/L) + 7.3353 (R2 = 0.9908). And the detection limit is 6.9 × 10-8 mol/L (3σ,where σ was the standard deviation of 11 parallel measurements of the blank solution).

|
Download:
|
| Fig. 3.(A) DPVs of a series of concentrations (0.1, 1, 2, 5, 10, 20, 50, 80 and 100 μmol/L) of CAP at MoS2/PANI/CPE in PBS. (B) The calibration plots of the reduced peak current versus different concentrations of CAP. | |
We studied the reproducibility through the detection of 1.0 × 10-5 mol/L CAP at ten different MoS2/PANI/CPEs. The relative standard deviation was about 4.82%,indicating an acceptable reproducibility. The stability of MoS2/PANI/CPE was also investigated by detecting the current response of 1.0 × 10-5 mol/L CAP at a storage period of two weeks,and it still retained 92% of the initial current,which indicting a good stability for detection of CAP using this electrochemical sensor.
4. ConclusionIn summary,we synthesized the thin-layered MoS2/PANI nanocomposite through the combination of ultrasonic exfoliation of bulk MoS2 and in situ polymerization of aniline,and fabricated a novel electrochemical sensor based on this nanocomposite for the sensitive detection of CAP. Due to the synergistic effect of MoS2 and PANI,the electrochemical sensor exhibited excellent performance for detection of CAP. Among all the modified electrodes,MoS2 (0.018 g)/PANI/CPE owns high sensitivity,a wide detection range and a low detection limit as well as good reproducibility and stability for detection of CAP. Besides,this MoS2/PANI-based electrochemical sensor could be further developed to a broad-spectrum analytic means for the detection of some conjugated aromatic molecules and widely used in electrochemical biosensing.
5. AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21275084 and 41476083),Doctoral Foundation of the Ministry of Education of China (No. 20113719130001),Scientific and Technical Development Project of Qingdao (No. 12-1-4-3-(23)-jch),and Outstanding Adult-Young Scientific Research Encouraging Foundation of Shandong Province (No. BS2012CL013).
| [1] | C.T. Kong, D.E. Holt, S.K. Ma, A.K.W. Lie, L.C. Chan, Effects of antioxidants and a caspase inhibitor on chloramphenicol-induced toxicity of human bone marrow and HL-60 cells, Hum. Exp. Toxicol. 19 (2000) 503-510. |
| [2] | L. Agüí, A. Guzmán, P. Yáñez-Sedeñ o, J.M. Pingarró n, Voltammetric determination of chloramphenicol in milk at electrochemically activated carbon fibre microelectrodes, Anal. Chim. Acta 461 (2002) 65-73. |
| [3] | P. Li, Y.M. Qiu, H.X. Cai, et al., Simultaneous determination of chloramphenicol, thiamphenicol, and florfenicol residues in animal tissues by gas chromatography/mass spectrometry, Chin. J. Chromatogr. 24 (2006) 14-18. |
| [4] | S.I. Kawano, H.Y. Hao, Y. Hashi, J.M. Lin, Analysis of chloramphenicol in honey by on-line pretreatment liquid chromatography-tandem mass spectrometry, Chin. Chem. Lett. 26 (2015) 36-38. |
| [5] | S. Teixeira, C. Delerue-Matos, A. Alves, L. Santos, Fast screening procedure for antibiotics in wastewaters by direct HPLC-DAD analysis, J. Sep. Sci. 31 (2008) 2924-2931. |
| [6] | W.R. Jin,X.Y.Ye, D.Q.Yu,Q.Dong,Measurement of chloramphenicol bycapillary zone electrophoresis following end-column amperometric detection at a carbon fiber micro-disk array electrode, J. Chromatogr. B: Biom. Sci. Appl. 741 (2000) 155-162. |
| [7] | M.C. Icardo, M. Misiewicz, A. Ciucu, J.V.G. Mateo, J.M. Calatayud, FI-on line photochemical reaction for direct chemiluminescence determination of photodegradated chloramphenicol, Talanta 60 (2003) 405-414. |
| [8] | R.R. Yang, J.L. Zhao, M.J. Chen, et al., Electrocatalytic determination of chloramphenicol based on molybdenum disulfide nanosheets and self-doped polyaniline, Talanta 131 (2015) 619-623. |
| [9] | N. Prabhakar, K. Arora, H. Singh, B.D. Malhotra, Polyaniline based nucleic acid sensor, J. Phys. Chem. B 112 (2008) 4808-4816. |
| [10] | T. Yang, Q.H. Li, X. Li, et al., Freely switchable impedimetric detection of target gene sequence based on synergistic effect of ERGNO/PANI nanocomposites, Biosens. Bioelectron. 42 (2013) 415-418. |
| [11] | H.S.S.R. Matte, A. Gomathi, A.K. Manna, et al., MoS2 and WS2 analogues of graphene, Angew. Chem. Int. Ed. 49 (2010) 4059-4062. |
| [12] | E. Benavente, M.A.S. Ana, F. Mendizábal, G. González, Intercalation chemistry of molybdenum disulfide, Coord. Chem. Rev. 224 (2002) 87-109. |
| [13] | B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti, A. Kis, Single-layer MoS2 transistors, Nat. Nanotechnol. 6 (2011) 147-150. |
| [14] | K. Chang, W.X. Chen, In situ synthesis of MoS2/graphene nanosheet composites with extraordinarily high electrochemical performance for lithium ion batteries, Chem. Commun. 47 (2011) 4252-4254. |
| [15] | L.C. Yang, S.N. Wang, J.J. Mao, et al., Hierarchical MoS2/polyaniline nanowires with excellent electrochemical performance for lithium-ion batteries, Adv. Mater. 25 (2013) 1180-1184. |
| [16] | L.R. Hu, Y.M. Ren, H.X. Yang, Q. Xu, Fabrication of 3D hierarchical MoS2/polyaniline and MoS2/C architectures for lithium-ion battery applications, ACS Appl. Mater. Interfaces 6 (2014) 14644-14652. |
| [17] | T. Yang, Q. Guan, X.H. Guo, et al., Direct and freely switchable detection of target genes engineered by reduced graphene oxide-poly (m-aminobenzenesulfonic acid) nanocomposite via synchronous pulse electrosynthesis, Anal. Chem. 85 (2013) 1358-1366. |
 2016, Vol.27
2016, Vol.27 


