The pyrimidinone skeleton has received considerable attention in recent years because of its existence in many natural or synthetic biologically active materials,and its derivatives have been applied to various pharmaceutical and biochemical fields [1, 2]. It is of great interest that,specifically functionalized pyrimidinone may possess specific biological properties,such as antihypertensive,antiviral,antifungal and anticancer activities [3, 4]. This has led to the development of various methods for the synthesis of pyrimidinone and its derivatives in the last few years.
Biginelli [5] reported that in concentrated hydrochloric acid,3,4-dihydropyrimidine-2(H)-ketones were generated through a ring condensation reaction of ethyl acetoacetate,aromatic aldehydes and urea,which was now widely known as the Biginelli-type reaction. Its operation is simple,but the yield is low (20%-50%). In 2005,Pan [6] first described an efficient method for the synthesis of pyrimidinones by a three-component condensation with aromatic aldehydes,cyclopentanone,and urea or thiourea as starting materials. Although the reaction could proceed smoothly,the use of stoichiometric amount of TMSCl as an additional reagent and mixed CH3CN/DMF as a reaction solvent were necessary to obtain the targets. In recent years,various synthetic methods for pyrimidine ketones have been developed,which can be roughly categorized as catalytic synthesis [7, 8, 9, 10],solid synthesis [11],microwave-promoted synthesis [12] and so on. However,these methods mainly related to the study of classical Biginelli reaction,and the synthesis of pyrimidine ketone and its derivatives were seldom reported [13].
Metal-catalyzed reactions [14] are recognized as attractive and environmentally benign methods in synthetic organic chemistry. In this area,remarkable progress has been made in the applications of lanthanide reagents as catalysts in organic synthesis recently [15]. Zhang [16] described a method for the synthesis of pyrimidinones using a three-component condensation with aromatic aldehydes,cyclopentanone,and urea catalyzed by ytterbium chloride. But the costly metal catalysts are not readily available and they cannot be recycled and reused. In addition,reagent wasting and long reaction time make this method less appealing.
Considering the characteristics mentioned above,the room temperature ionic liquids have been described as one of the most promising new reaction mediums [17] because of their relatively benign characters,low volatility,thermal stability,efficiency as a catalyst and promoter,and reusability [18]. Bronsted acid ionic liquids (ILs) containing acidic protons that are adjustable to exhibit desired catalytic activity. On the basis of previous pioneering work,a novel environment-friendly ionic liquid [C3SO3HDoim]HSO4 was designed to catalyze the Biginelli-type condensation reaction,which exhibited dramatically efficiency in the course of the reaction with its sulfonic functional groups and long carbon chain. In addition,[C3SO3HDoim]HSO4 can be successfully recovered and reused for several times.
2. ExperimentalAll reagents were of analytical grade and were purchased from commercial sources and used without any further purification. Melting points were determined on a Thomas Hoover capillary apparatus and were uncorrected. 1 H NMR (500 MHz) was recorded on a Bruker 500 spectrometer with tetramethylsilane (TMS) as an internal standard. Mass spectra was recorded on an Agilent technologies 6110 quadrupole LC/MS equipped with an electrospray ionization (ESI) probe operating in the positive ion mode. Yields refer to the isolated yields of the products after purification. All starting chemicals were commercially available. [HMim]X (X = HSO4,HNO3),[TEPSA]HSO4 and [C3SO3HMim]HSO4 were prepared according to literature procedures [19, 20, 21].
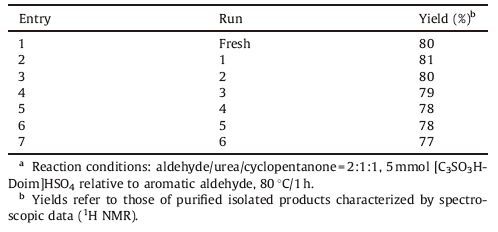
2.1. Preparation of [C3SO3HDoim]HSO4The synthetic process of [C3SO3HDoim]HSO4 was shown in Scheme 1. First,imidazole (6.8 g,0.1 mol) was dissolved in 100 mL of ethanol sodium ethoxide. The mixture was heated to 70 ℃ and kept at this temperature with stirring for 8 h under reflux [22]. Then an equal mole of 1-bromododecane was added and the system was allowed to stir for 8 h under reflux. The white precipitate was filtered off and the filtrate was extracted three times by diethyl ether followed by vacuum distillation to remove organic solvents to obtain N-dodecyl imidazole,a yellow viscous liquid at room temperature. N-Dodecyl imidazole was characterized by LC/MS.
|
Download:
|
| Scheme 1.he synthesis of 1-dodecyl-3-(3-sulfopropyl)-imidazolium hydrogen sulfate. | |
Second,an equal mole of 1,3-propanesultone was added dropwise over a period of 10 min under stirring in an ice bath [23],after which the mixture was heated to 70 ℃ and kept at this temperature with stirring for 10 h,followed by the addition of an equal mole of sulfur acid (98%) with stirring. The mixture was then warmed to 90 ℃ and kept at this temperature for 2 h [21]. After that,diethyl ether was added and 1-dodecyl-3-(3-sulfopropyl)- imidazolium hydrogen sulfate ([C3SO3HDoim]HSO4) was precipitated as a highly viscous and pale-yellow oily liquid. The product was dried under vacuum at 50 ℃ for 6 h giving a yield of 98%-99%. [C3SO3HDoim]HSO4 was characterized by IR,1H NMR,13C NMR and LC/MS.
2.2. Typical procedure for the synthesis of pyrimidinonesA mixture of aromatic aldehydes (50 mmol),cyclopentanone (2.1 g,25 mmol),urea or thiourea (25 mmol) and [C3SO3HDoim] HSO4 (5 mmol) was well stirred at 80 ℃ for a given time. After that,the system was cooled to room temperature. The pure product was isolated by filtering through a Bu¨ chner funnel and washed with water,diethyl ether and acetone,followed by crystallization from ethanol,and then dried to give the crystalline or powdered products [6].
The spectral data of known compounds characterized by 1H NMR were found to be identical with those reported in the literature [16, 24].
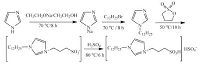
3. Results and discussionThe experimental process could be easily achieved with simple operation under solvent-free conditions. Four ILs were prepared and used as catalysts for the Biginelli-type reaction,including [HMim]HSO4,[TEPSA]HSO4,[C3 SO3HMim]HSO4,[C3SO3HDoim]HSO4. The structures were shown in Fig. 1.
|
Download:
|
| Fig. 1.Four kinds of ionic liquids. | |
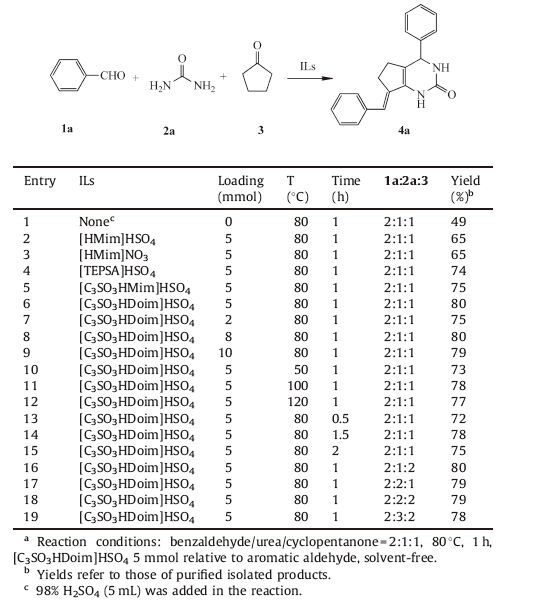
The catalytic performance of different ILs was summarized in Table 1. During the experimental process,a mixture of three reagents,benzaldehyde,cyclopentanone,and urea was stirred at a suitable temperature in air in the presence of IL,which acted as a model reaction to optimize reaction conditions. As can be seen,[C3SO3HDoMim]HSO4 was the most efficient one,giving the highest yield.
|
|
Table 1 Optimization of the reaction conditions.a |
As shown in Table 1 (entries 1-6),in the absence of IL,the yield was only 49% using sulfuric acid as catalyst. The IL,[C3SO3HDoim] HSO4,was found to give the best result at 80 ℃ compared with other imidazolium salt-based ILs such as [HMim]HSO4 and [C3SO3HMim]HSO4,or hyamine-based ILs. The possible reason for this was that [C3SO3HDoim]HSO4 with a distinct sulfonic functional group and long carbon chain could make it soluble in organic phase,which was beneficial for the interactions between catalyst and substrates. On the other hand,imidazole cation could form coordination bonds with the carbonyl oxygen,which performed better than the hyamine ion. With [C3SO3HDoim]HSO4 as a model catalyst,the screening of catalyst loading was then carried out. The result revealed that 5 mmol% was the most suitable proportion,more loading or less could not enhance the product yield (entries 6-9). Reaction temperature significantly affected the reaction. The rise of the temperature from 50 ℃ to 80 ℃ led to an increased yield from 73% to 80% (entries 6 and 10),while a decreased yield was obtained when the temperature was over 100 ℃ (entries 11 and 12). Then the impact of reaction time on yield (entries 6 and 13-15) was investigated and the highest yield was obtained after 1 h. These results indicated that lower temperature and shorter reaction time may result in incomplete reaction while overheating and prolonged time led to complicated side reactions.
The ratio of benzaldehyde,urea and cyclopentanone should be 2:1:1 to produce pyrimidinones in theory. To explore the influence of the ratio on this reaction,we attempted to modify the ratio in various ways. Corresponding control reactions were performed and the results indicated that even when the amount of cyclopentanone or urea was doubled,there is almost no increase in yield (entries 6 and 16-19). In other words,excessively used cyclopentanone and urea barely enhanced the yield with the participation of [C3SO3HDoim]HSO4,which is contrasted to the research of Zhang [16].
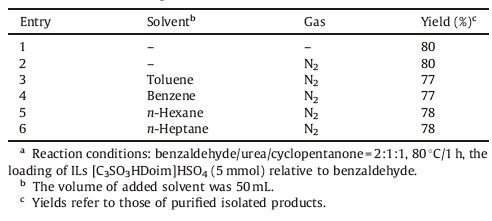
In addition,using [C3SO3HDoim]HSO4 as a catalyst,the effect of gas and solvent on the reaction was also investigated,as shown in Table 2. N2 was used as a protection gas to screen the reaction conditions compared with air (entries 1 and 2). The results showed that N2 protection could increase the yield. In other words,materials in air containing CO2,O2,H2O,etc. did not have an influence on the Biginelli-type reaction. Besides,four common organic reagents,such as toluene,benzene,n-hexane,n-heptane,were chosen as solvents in this reaction (entries 3-6). It was found that solvent-free condition under atmosphere gave the best result with a yield of 80% after 1 h. A probable reason would be that solvation effect hindered the catalytic effect of IL. So N2 protection and organic solvent could be avoided to afford a simple and green process. Thus the reaction worked well while being exposed to air under solvent-free conditions.
|
|
Table 2 The effect of solvent and gas on the reaction.a |
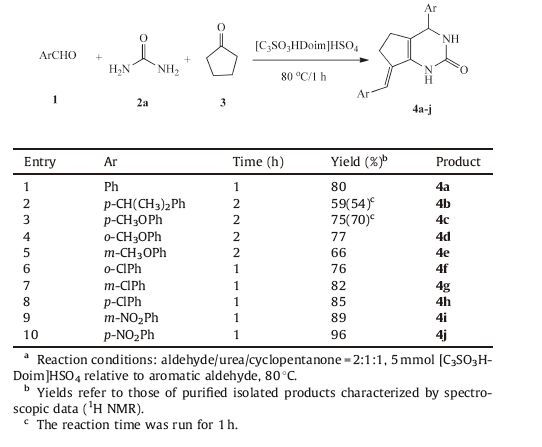
In order to examine the scope and generality of this procedure,we extended the methodology to different aromatic aldehydes. Several substituted aromatic aldehydes were applied to this condensation reaction with cyclopentanone and urea to produce corresponding pyrimidinones. The results were summarized in Table 3. Aromatic aldehydes with both electron donating (entries 2-5) and electron withdrawing groups (entries 6-10) could participate this reaction effectively. Aldehydes with electron withdrawing groups reacted with cyclopentanone and urea smoothly to give the corresponding products in higher yields compared with the ones with electron donating groups. So we attempted to prolong the reaction time moderately for aldehydes with electron donating groups to increase the yields as much as possible (entries 2-5). In addition,the high stereo-hindrance effect was another vital reason for the relatively low yield as seen in the case of para-isopropylbenzaldehyde. We found that the nature and position of substitution on the aryl ring did have an influence on reactivity. Apparently,substrates with strong electron-withdrawing group (entries 9 and 10) gave evidently increased yields,compared with the weak ones (entries 6-8). In addition,the parasubstituted aldehyde could afford a higher yield than the meta- and ortho-substituted ones. So it was proposed that as for the attack of carbon in aldehyde group by nucleophilic species,benzaldehyde with electron-withdrawing substituent group was easier than that with the electron-donating one.
|
|
Table 3 Synthesis of pyrimidinones 4(a–j) from aromatic aldehydes, cyclopentanone and urea.a |
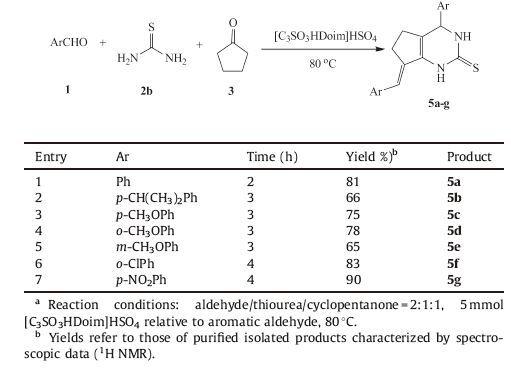
Considering the widespread use of sulfur-containing intermediates in the medical fields [24],thiourea was used in place of urea in this reaction. Under the optimized conditions,the synthesis of pyrimidinones with aromatic aldehydes,cyclopentanone and thiourea was achieved,as shown in Table 4. In view of electronic effect,sulfur exhibited stronger electron-donating ability than oxygen. Thus,unlike urea,reactions of thiourea proceeded smoothly to give targeted products in yields ranging from 66% to 90% with high purity. But reaction time would be prolonged to achieve a higher yield. We believed that expanding the reaction from urea to thiourea would be meaningful to synthesize biologically active pyrimidinone scaffolds.
|
|
Table 4 Synthesis of pyrimidinones 5(a–g) from aromatic aldehydes, cyclopentanone and thiourea.a |
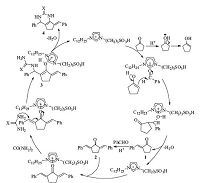
On the basis of our experimental results and literature reports about the synthesis of pyrimidinones [16, 25],and previous studies of ILs-catalyzed condensation reactions [26, 27],a possible mechanism for the synthesis of pyrimidinones catalyzed by [C3SO3HDoim]HSO4 was proposed as shown in Scheme 2. [C3SO3HDoim]HSO4 showed remarkable reactivity as an organicphase catalyst,which considerably accelerated the reactions. It was probably due to its long chain with twelve carbons that allowed it to dissolve in the organic phase more easily compared with [C3SO3HMim]HSO4 containing only one methyl. Thus,a hydrogen proton from [C3SO3HDoim]HSO4 combined with ketonic oxygen in cyclopentanone to produce an enol form. Then the crossaldol condensation of benzaldehyde with the enol form took place to generate keto-aldehyde 1 with the assistance of [C3SO3HDoim] HSO4,which was similar to the Yb(OTf)3-catalyzed process reported by Wang [28]. Afterwards,keto-aldehyde 1 further reacted with another benzaldehyde resulting in the formation of keto-aldehyde 2 in the same way. Then compound 3 was generated through a Michael addition of urea or thiourea with keto-aldehyde 2,followed by the removing a H2O molecule and cyclizing to form the targeted compound 4. When one round of condensation reaction was finished,[C3SO3HDoim]HSO4 was recovered and catalyzed the reaction over and over.
|
Download:
|
| Scheme 2.The possible mechanism for the synthesis of pyrimidinones catalyzed by [C3SO3HDoim]HSO4 (X = O, S). | |
At last,the recycling performance of [C3SO3HDoim]HSO4 was also investigated using the model reaction. After the separation of the products,the solvents in the filtrate were moved through rotary evaporation to recover [C3SO3HDoim]HSO4,which could be reused without further purification. The data listed in Table 5 showed that the [C3SO3HDoim]HSO4 could be reused at least 7 times without obvious reduction of the catalytic activity. Compared with the traditional catalysts,the easy recycling performance is also an attractive property of the [C3SO3HDoim] HSO4 for the environmental protection and economic reasons. The reason for the minimal loss in yield could be that the ILs run off with filtration and purification of the products. Upon our research,it can be seen that this ILs-catalyzed condensation reaction for the synthesis of pyrimidinones constituted an efficient,convenient,eco-friendly and most importantly recyclable system. Firstly,no poisonous auxiliary solvents were injected into the reaction,and the purification of the products was just performed through filtration,washing and crystallization. Second,the reaction worked well while being exposed to the air,so the steps of drying and protecting were avoided. In addition,reusability of ILs led to an economical and environmental benign process.
|
|
Table 5 Recyclability of recovered [C3SO3HDoim]HSO4.a |
In conclusion,the present procedure using a novel functionalized IL [C3SO3HDoim]HSO4 as catalyst,provides a very simple and efficient methodology for the synthesis of pyrimidinone and its derivatives through the condensation of aromatic aldehydes,cyclopentanone and urea or thiourea. In addition to short reaction time,low energy consumption,high yields,and reusability of ILs,no hazardous organic solvents are used in the entire processes including workup and purification. These eco-friendly features provide an attractive method to synthesize pyrimidinones.
| [1] | K.S. Atwal, G.C. Rovnyak, B.C. O'Reilly, J. Schwartz, Substituted 1,4-dihydropyrimidines. 3. Synthesis of selectively functionalized 2-hetero-1,4-dihydropyrimidines, J. Org. Chem. 54 (1989) 5898-5907. |
| [2] | C.O. Kappe, W.M.F. Fabian, M.A. Semones, Conformational analysis of 4-aryldihydropyrimidine calcium channel modulators. A comparison of ab initio, semiempirical and X-ray crystallographic studies, Tetrahedron 53 (1997) 2803-2816. |
| [3] | A.D. Patil, N.V. Kumar, W.C. Kokke, et al., Novel alkaloids from the sponge Batzella sp.: inhibitors of HIV gp120-human CD4 binding, J. Org. Chem. 60 (1995) 1182-1188. |
| [4] | H.I. EI-Subbagh, S.M. Abu-Zaid, M.A. Mahran, et al., Synthesis and biological evaluation of certain α,β-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents, J. Med. Chem. 43 (2000) 2915-2921. |
| [5] | P. Biginelli, Aldureides of ethylic acetoacetate and ethylic oxalacetate, Gazz. Chim. Ital. 23 (1893) 360-416. |
| [6] | Y.L. Zhu, S.L. Huang, Y.J. Pan, Highly chemoselective multicomponent Biginellitype condensations of cycloalkanones, urea or thiourea and aldehydes, Eur. J. Org. Chem. 11 (2005) 2354-2367. |
| [7] | (a) E.H. Hu, D.R. Sidler, U.H. Dolling, Unprecedented catalytic three component one-pot condensation reaction: an efficient synthesis of 5-alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones, J. Org. Chem. 63 (1998) 3454-3457;(b) P.P. Warekar, G.B. Kolekar, M.B. Deshmukh, P.V. Anbhule, An efficient and modified Biginelli-type synthesis of 3,4-dihydro-1H-indeno[1,2-d] pyrimidine-2,5-dione using phosphorous pentoxide, Synth. Commun. 44 (2014) 3594-3601. |
| [8] | (a) J. Lu, H. Ma, Iron(Ⅲ)-catalyzed synthesis of dihydropyrimidinones. Improved conditions for the Biginelli reaction, Synlett 207 (2000) 63-64;(b) J. Lu, Y. Bai, Z. Wang, B. Yang, H. Ma, One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using lanthanum chloride as a catalyst, Tetrahedron Lett. 41 (2000) 9075-9078;(c) E. Pair, V. Levacher, J.F. Briere, Organocatalyzed multicomponent synthesis of pyrazolidinones: meldrum's acid approach, RSC Adv. 46 (2015) 46267-46271. |
| [9] | Y. Ma, C. Qian, L. Wang, M. Yang, Lanthanide triflate catalyzed Biginelli reaction. One-pot synthesis of dihydropyrimidinones under solvent-free conditions, J. Org. Chem. 65 (2000) 3864-3868. |
| [10] | B.C. Ranu, A. Hajra, U. Jana, Indium(Ⅲ) chloride-catalyzed one-pot synthesis of dihydropyrimidinones by a three-component coupling of 1,3-dicarbonyl compounds, aldehydes, and urea: an improved procedure for the Biginelli reaction, J. Org. Chem. 65 (2000) 6270-6272. |
| [11] | (a) P. Wipf, CunninghamF A., A solid phase protocol of the Biginelli dihydropyrimidine synthesis suitable for combinatorial chemistry, Tetrahedron Lett. 36 (1995) 7819-7822;(b) A. Studer, P. Jeger, P. Wipf, P. Curran, Fluorous synthesis: fluorous protocols for the Ugi and Biginelli multicomponent condensations, J. Org. Chem. 62 (1997) 2917-2924. |
| [12] | (a) H.A. Stefani, P.M. Gatti, 3,4-Dihydropyrimidin-2(1H)-ones: fast synthesis under microwave irradiation in solvent free conditions, Synth. Commun. 30 (2000) 2165-2173;(b) C.O. Kappe, D. Kumar, R.S. Varma, Microwave-assited high-speed parallel synthesis of 4-aryl-3,4-dihydropyrimidin-2(1H)-ones using a solventless Biginelli condensation protocol, Synthesis 10 (1999) 1799-1803. |
| [13] | (a) B.B. Snider, J. Chen, A.D. Patil, A. Freyer, Synthesis of the tricyclic portions of batzelladines A, B and D. Revision of the stereochemistry of batzelladines A and D, Tetrahedron Lett. 37 (1996) 6977-6980;(b) A.V. RamaRao, M.K. Gujar, J. Vasudevan, An enantiospecific synthesis of the tricyclic guanidine segment of the anti-HIV marine alkaloid batzelladine A, J. Chem. Soc., Chem. Commun. 26 (1995) 1369-1370;(c) Z. Wang, L. Xu, C. Xia, H. Wang, Novel Biginelli-like three-component cyclocondensation reaction: efficient synthesis of 5-unsubstituted 3,4-dihydropyrimidin-2(1H)-ones, Tetrahedron Lett. 45 (2004) 7951-7953;(d) B. Liang, X. Wang, J.X. Wang, Z. Du, New three-component cyclocondensation reaction: microwave-assisted one-pot synthesis of 5-unsubstituted-3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions, Tetrahedron 63 (2007) 1981-1986. |
| [14] | D.M. D'Souza, T.J.J. Muller, Multicomponent syntheses of heterocycles by transition-metal catalysis, Chem. Soc. Rev. 36 (2007) 1095-1108. |
| [15] | (a) S. Kobayashi, M. Sugiura, H. Kitagawa, W.W.L. Lam, Chem inform abstract: rare-earth metal triflates in organic synthesis, Chem. Rev. 102 (2002) 2227-2302;(b) M. Shibasaki, S. Matsunaga, Design and application of linked-BINOL chiral ligands in bifunctional asymmetric catalysis, Chem. Soc. Rev. 35 (2006) 269-279. |
| [16] | H. Zhang, Z. Zhou, Z. Yao, F. Xu, Q. Shen, Efficient synthesis of pyrimidinone derivatives by ytterbium chloride catalyzed Biginelli-type reaction under solventfree conditions, Tetrahedron Lett. 50 (2009) 1622-1624. |
| [17] | K.R. Seddon, Ionic liquids for clean technology, J. Chem. Technol. Biotechnol. 68 (1997) 351-356. |
| [18] | (a) T. Welton, Room-temperature ionic liquids. Solvents for synthesis and catalysis, Chem. Rev. 99 (1999) 2071-2084;(b) P. Wasserscheid, W. Keim, Ionic liquids-new "solutions" for transition metal catalysis, Angew. Chem. Int. Ed. 39 (2000) 3772-3789;(c) J.S. Wilkes, A short history of ionic liquids-from molten salts to neoteric solvents, Green Chem. 4 (2002) 73-80;(d) V.I. Parvulescu, C. Hardacre, Catalysis in ionic liquids, Chem. Rev. 107 (2007) 2615-2665. |
| [19] | C. Chiappe, E. Leandri, M. Tebano, [Hmim] [NO3] —an efficient solvent and promoter in the oxidative aromatic chlorination, Green Chem. 8 (2006) 742-745. |
| [20] | D. Fang, J. Luo, X.L. Zhou, Z.L. Liu, One-pot green procedure for Biginelli reaction catalyzed by novel task-specific room-temperature ionic liquids, J. Mol. Catal., A: Chem. 274 (2007) 208-211. |
| [21] | S.F. Chai, L.S. Wang, G.Q. Yan, Y. Li, Solubilities of 1-methyl-3-(3-sulfopropyl)-imidazolium hydrogen sulfate in selected solvents, J. Chem. Eng. Chin. 18 (2010) 1008-1012. |
| [22] | H.Z. Zhi, C.X. Lv, Q. Zhang, et al., A new PEG-1000-based dicationic ionic liquid exhibiting temperature-dependent phase behavior with toluene and its application in one-pot synthesis of benzopyrans, Chem. Commun. 20 (2009) 2878-2880. |
| [23] | A.C. Cole, J.L. Jensen, I. Ntai, et al., Novel bronsted acidic ionic liquids and their use as dual solvent-catalysts, J. Am. Chem. Soe. 124 (2002) 5962-5963. |
| [24] | A. Yoshimura, A. Nomoto, A. Ogawa, Rhodium-catalyzed hydrothiolation of alkynes with thiols for construction of sulfur-containing π-conjugated systems, Res. Chem. Intermediat. 40 (2014) 2381-2389. |
| [25] | (a) C.O. Kappe, A reexamination of the mechanism of the Biginelli dihydropyrimidine synthesis. Support for an N-acyliminium ion intermediate, J. Org. Chem. 62 (1997) 7201-7204;(b) C.O. Kappe, Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog, Acc. Chem. Res. 33 (2000) 879-888. |
| [26] | D. Saha, A. Saha, B.C. Ranu, Remarkable influence of substituent in ionic liquid in control of reaction: simple, efficient and hazardous organic solvent free procedure for the synthesis of 2-aryl benzimidazoles promoted by ionic liquid, [pmim] BF4, Green Chem. 11 (2009) 733-737. |
| [27] | (a) M.J. Earle, S.P. Katdare, K.R. Seddon, Paradigm confirmed: the first use of ionic liquids to dramatically influence the outcome of chemical reactions, Org. Lett. 6 (2004) 707-710;(b) B.C. Ranu, S. Banerjee, The dramatic influence of a task-specific ionic liquid, [bmIm] OH, in michael addition of active methylene compounds to conjugated ketones, carboxylic esters, and nitriles, Org. Lett. 7 (2005) 3049-3052. |
| [28] | L. Wang, J. Sheng, H. Tian, et al., A convenient synthesis of α,α'-bis(substituted benzylidene)cycloalkanones catalyzed by Yb(OTf)3 under solvent-free conditions, Synthesis 18 (2004) 3060-3064. |
 2016, Vol.27
2016, Vol.27