b Department of Pharmaceutical Biotechnology, School of Pharmacy, Zanjan University of Medical Sciences, Zanjan, Iran;
c Department of Medicinal Chemistry, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran;
d Phytochemistry Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Compounds derived from isatin (1H-indole-2,3-dione) have long been known to possess versatile biological activities. Like indole-based derivatives,isatins have been studied for their potential antimicrobial [1],antiviral [2, 3, 4] and antiproliferative [5, 6, 7] activities. Isatin is also a part of many heterocyclic natural products of biological interest [8, 9]. Thiosemicarbazone derivatives of N-methylisatin (Methisazone) have long been used as antiviral agents against active variola and vaccinia viruses [10].
In several studies aimed at developing antibacterial agents,isatin has been considered as the core fragment. By different chemical modifications including Schiff base formation,N-alkylation/ acylation [1, 11],Mannich base synthesis [12, 13] and metal complexation [14, 15],research groups have reported a variety of chemical entities,many of which showed promising activity in antibacterial screenings. Some examples of isatin derivatives with reported antimicrobial activity have been disclosed in Fig. 1.
|
Download:
|
| Fig. 1.Bioactive isatin derivatives. (I) Methisazone [2]; (II) modified methisazone derivatives as antiviral protease inhibitors [3]; (III) Mannich bases of isatin as anticancer agents, X = NH, S [5]; (IV) isatinhydrazones with activity against multidrug resistant cells, X = O, S, Ar: substituted phenyl [6]. | |
On the other hand,hydrazone derivatives have been among the most interesting class of compounds especially in antimicrobial drug research. In a series of focused studies on (thio)semicarbazones of aromatic aldehydes/ketones,we have found some potent antimycobacterial,antifungal and anticancer compounds among hydrazones derived from substituted indole-3-carboxaldehydes and benzaldehydes [16, 17, 18, 19, 20]. Our results on indole-based derivatives encouraged us to extend our studies to isatin derivatives,which could be considered as a close analog of their indole counterparts.
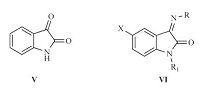
Based on the above considerations and using the molecular hybridization strategy,in the present study a series of compounds containing isatin and hydrazone fragments were designed (Fig. 2). The final compounds were prepared starting from 5-substituted isatins and appropriate hydrazide derivatives. In addition to (thio)semicarbazide,the other starting amines/hydrazides were in turn selected based on the previous reports indicating their antibacterial activity. Evaluation of the existing reports shows that small molecules derived from hydantoin [21, 22],4-hydroxybenzohydrazide [23] and isoniazid [24] have demonstrated promising antibacterial activity.
|
Download:
|
| Fig. 2.(V) Isatin; (VI) general structure of the synthesized derivatives. | |
We found it interesting to assess whether molecular hybrids containing isatin and bioactive amines/hydrazides could demonstrate promising activity against pathogenic bacteria. To this end,34 Schiff base derivatives of 5-substituted isatins were prepared and evaluated against 6 clinically important bacterial strains of both gram-positive and gram-negative class.
2. ExperimentalMelting points were measured using an Electrothermal 9200 apparatus and were uncorrected. The proton nuclear magnetic resonance (1H NMR) spectra were measured on a 400 MHz Bruker Avance DRX spectrometer. Compounds were dissolved in MDSO-d6 or CDCl3 and TMS was used as an internal standard. The 13C NMR spectra were also recorded on the same spectrometer and the compounds were dissolved in d6-DMSO. The infrared spectra were obtained using a Bruker Tensor 27 FTIR spectrophotometer and KBr was used as a diluent. ESI-MS spectra were obtained using Agilent 6410 Triple Quad LC/MS. The derivatives were analyzed for C,H,N,S by a Costech model 4010 and the obtained values were in agreement with the proposed structures within -0.4% of the theoretical values. The spectral characterization data of the synthesized intermediates and final compounds has been provided in the Supporting information.
2.1.1. General procedure for the synthesis of Schiff basesEquimolar amines and 5-substituted isatins (1 mmol of each) were added to 96% w/w ethanol (20 mL) containing 8 drops of glacial acetic acid. The mixture was heated under reflux for 5 h and then cooled to room temperature. The resulting solid was collected by filtration,washed with cold ethanol and dried in open air. The derivatives thus prepared had sufficient analytical purity. The synthesis and spectral characterizations of compounds 4b [25],4c [26],5c [25],6a [27],6b,6c and 6d [28] have been previously reported in the literature.
2.1.2. General procedure for the synthesis of N-benzylisatins 8-10To a flask containing acetonitrile (15 mL) were added isatin (294 mg,2 mmol),K2CO3 (332 mg,2.4 mmol) and KI (66 mg,0.4 mmol). Stirring was started and after 5 min,fluorobenzyl chlorides (433 mg,3 mmol) were added dropwise. After 3 h of reflux,the mixture was cooled to room temperature and filtered. The filtrate was concentrated under reduced pressure and the resulting oil was solidified upon the addition of n-hexane. The solid was recrystallized in water to afford the titled compounds.
2.2. Evaluation of antibacterial activityAntimicrobial activity of the synthesized derivatives was evaluated using a microtiter plate method. The bacterial strains included Pseudomonas aeruginosa (ATCC 27853),Escherichia coli (ATCC 25922),Enterococcus faecalis (ATCC 29212),Staphylococcus aureus (ATCC 25923) and Methicillin resistant S. aureus (MRSA,ATCC 43300). Amikacin was used as a standard antibiotic. The detailed procedure has been described in our previous work [29].
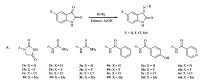
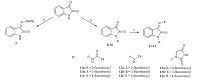
3. Results and discussion 3.1. ChemistryThe final imine/hydrazone derivatives were prepared via reacting 5-substituted isatins with the selected amines including 1-aminohydantoin,semicarbazide,thiosemicarbazide,benzohydrazide,4-hydroxybenzohydrazide and isoniazid (Scheme 1). The components were added to ethanol with a few drops of acetic acid as a catalyst. The Schiff base products were thus prepared in good yields and acceptable purity. For compounds 11-13,an additional step was required,which was the N-benzylation of isatin using fluorinated benzyl chlorides (Scheme 2). This reaction was performed using potassium carbonate in acetonitrile. Compounds 8,9 and 10 were then reacted with 3 different hydrazides following the above mentioned procedure. All the derivatives were analyzed using IR,NMR,Mass spectrometry and also elemental analysis. In the 1H NMR spectra of the derivatives,isatin hydrogens appeared as two sets of doublets (at ca. 6.93 and 7.59 ppm) and two sets of triplets (at ca. 7.10 and 7.36 ppm). In the case of 5-substituted isatins,one singlet and two sets of doublets in the aromatic region were observed. This pattern was helpful to recognize the peaks corresponding to the hydrazide and N-fluorobenzyl fragments. In the IR spectra,absorptions related to carbonyl functions of isatin and hydrazide moieties were the most intense ones appearing at 1680-1730 cm-1. The mass of the derivatives was also confirmed by ESI-MS,where values related to hydrogen and/or sodium adducts of the intact molecule were observed.
|
Download:
|
| Scheme 1.Synthetic route to Schiff bases of 5-substituted isatins. | |
|
Download:
|
| Scheme 2.Synthetic route to compound 7 and Schiff bases of N-arylmethylisatin 11a-13c. Reagents and conditions: (a) phenylhydrazine, ethanol 96% (w/w), acetic acid; (b) fluorinated benzyl chlorides, K2CO3, KI, acetonitrile; (c) compounds 8-10, ethanol 96% (w/w), acetic acid. | |
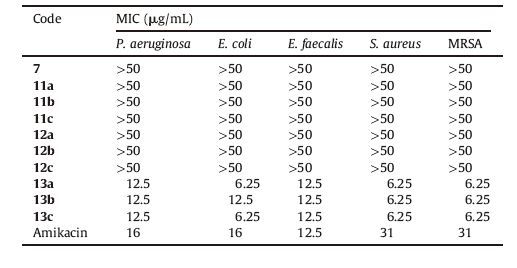
The in vitro antibacterial activity of the synthesized derivatives was evaluated against 6 clinically important bacterial strains from both gram positive and gram negative groups (Table 1). Most interestingly,the tested derivatives exhibited the highest activity on P. aeruginosa among which compounds 2d,3b,5c and 6a were the most potent ones (MIC = 6.25 μg/mL). This is an important finding since P. aeruginosa is a highly opportunistic pathogen and compounds with activity against this pathogenic bacterium are of special value. Further research and optimizations on these compounds may ultimately lead to the discovery of promising therapeutic agents.
|
|
Table 1 Antibacterial activity of isatin Schiff bases 1a–6d. |
As a general view,the (thio)urea-based hydrazone derivatives 1a-3d were more active than aroyl-based derivatives 4a-6d. This observation should be considered in designing new isatinhydrazones,especially when a broad spectrum of antibacterial activity is desired. It is also worth mentioning that among the bacterial strains evaluated,S. aureus and MRSA were the most resistant to the hydrazone derivatives.
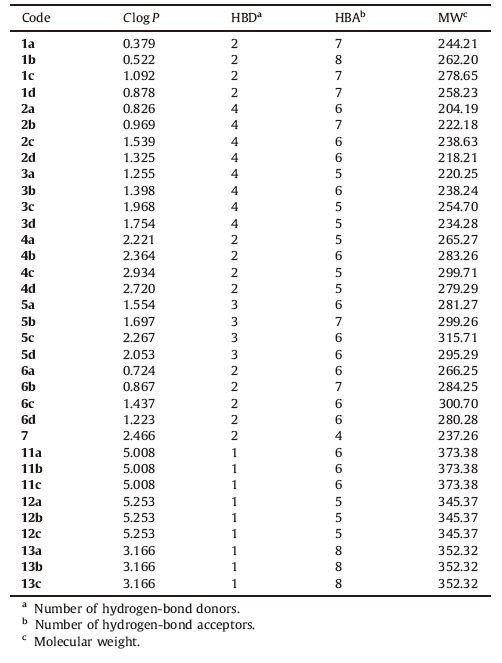
Despite their moderate activity,hydantoin derivatives 1a-1d were active against all the tested strains. This implies that introduction of hydantoin moiety provides the broader spectrum of antibacterial activity,though further optimization efforts are needed to improve their potency. The other interesting finding is that among the derivatives with a fluorobenzyl substitution at the isatin N - 1 position (11a-13c),the new benzylic substituent has been tolerated only in the hydantoin class (as in 13a-13c). As can be seen in Table 2,the N - 1 benzylation of isatin did not lead to any active compounds among benzoylhydrazones (11a-11c) and phenylhydrazones (12a-12c). This observation provides a new opportunity to optimize the potency of hydantoin derivatives through further structural manipulations including the introduction of new substituents at the isatin N - 1 position. This is the topic of our ongoing studies.
|
|
Table 2 Antibacterial activity of compounds 7 and N-fluorobenzylisatin derivatives 11a– 13c. |
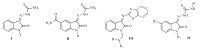
According to the Lipinski’s rule of five [30],a number of molecular properties play an important role in the optimum oral bioavailability and "drug-likeness" of small molecules. These properties include: number of hydrogen bond donors (should be less than 5); number of hydrogen bond acceptors (should be less than 10); lipophilicity (C log P value should be smaller than 5); and molecular weight (should be less than 500). These properties for the synthesized isatin derivatives were calculated and presented in Table 3. Among the various physicochemical parameters,lipophilicity has been the most evaluated one in the studies on antibacterial isatins [1]. According to the table,the C log P values of the most potent isatins fall between 0.72 and 2.27. Therefore,with regard to compounds 11a-12d,it could be hypothesized that in addition to steric hindrance,their highly lipophilic character might have a contribution to their loss of activity. This however must be further evaluated in the future studies through the introduction of substituents that lead to a wide range of C log P values.
|
|
Table 3 Calculated molecular properties related to the Lipinski’s rule of five. |
It is also important to note that the MW of the potent derivatives in this study is low enough to allow further molecular expansions in the future studies,meaning that more complex derivatives could still have theMWbelow 500. Finally,in a general view,all the synthesized derivatives conform to the limitations of Lipinski’s rule of five.
4. ConclusionIn summary,we evaluated the antibacterial activity of isatinbased hydrazone derivatives,which provided valuable clues for the further lead optimization studies. The most interesting observation was the promising activity of the tested hydrazones against P. aeruginosa as a clinically important strain. However,the derivatives did not exhibit a pronounced activity againstMRSA. Incorporation of (thio)urea fragments as amine components (which include semicarbazide,thiosemicarbazide and 1-aminohydantoin) led to an improved and broader spectrum of antibacterial activity. Among them,1-aminohydantoin imines 1a-1d were interesting not only because of their broad spectrum of activity,but also because their N - 1 benzyl analogs were still active. The latter is an important finding since it provides the opportunity to conduct new lead optimization studies and design new isatin-hydantoin derivatives with improved potency and spectrum of activity.
5. AcknowledgmentThe authors would like to express their appreciation to the research deputy of Zanjan University of Medical Sciences and Zanjan Institute for Advanced Studies in Basic Sciences for granting access to the NMR facility.
6. Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.10.027.
| [1] | P. Pakravan, S. Kashanian, M.M. Khodaei, F.J. Harding, Biochemical and pharmacological characterization of isatin and its derivatives: from structure to activity, Pharmacol. Rep. 65 (2013) 313-335. |
| [2] | M.C. Pirrung, S.V. Pansare, K.D. Sarma, K.A. Keith, E.R. Kern, Combinatorial optimization of isatin-β-thiosemicarbazones as anti-poxvirus agents, J. Med. Chem. 48 (2005) 3045-3050. |
| [3] | L. Zhou, Y. Liu, W.L. Zhang, et al., Isatin compounds as noncovalent SARS coronavirus 3C-like protease inhibitors, J. Med. Chem. 49 (2006) 3440-3443. |
| [4] | S.T. Xue, L.L. Ma, R.M. Gao, Y.H. Li, Z.R. Li, Synthesis and antiviral activity of some novel indole-2-carboxylate derivatives, Acta Pharm. Sin. B 4 (2014) 313-321. |
| [5] | A.T. Taher, N.A. Khalil, E.M. Ahmed, Synthesis of novel isatin-thiazoline and isatinbenzimidazole conjugates as anti-breast cancer agents, Arch. Pharmacal. Res. 34 (2011) 1615-1621. |
| [6] | M.D. Hall, N.K. Salam, J.L. Hellawell, et al., Synthesis, activity, and pharmacophore development for isatin-β-thiosemicarbazones with selective activity toward multidrug-resistant cells, J. Med. Chem. 52 (2009) 3191-3204. |
| [7] | Z.L. Song, M.N. Wang, A. Zhang, Alectinib: a novel second generation anaplastic lymphoma kinase (ALK) inhibitor for overcoming clinically-acquired resistance, Acta Pharm. Sin. B 5 (2015) 34-37. |
| [8] | P. Zhang, X.M. Li, J.N. Wang, X. Li, B.G. Wang, Prenylated indole alkaloids from the marine-derived fungus Paecilomyces variotii, Chin. Chem. Lett. 26 (2015) 313-316. |
| [9] | Z.G. Li, G.Q. Dong, S.Z. Wang, et al., Optical evodiamine derivatives: asymmetric synthesis and antitumor activity, Chin. Chem. Lett. 26 (2015) 267-271. |
| [10] | M. Bray, Pathogenesis and potential antiviral therapy of complications of smallpox vaccination, Antiviral Res. 58 (2003) 101-114. |
| [11] | Y. Wang, F.Y. Chan, N. Sun, et al., Structure-based design, synthesis, and biological evaluation of isatin derivatives as potential glycosyltransferase inhibitors, Chem. Biol. Drug Des. 84 (2014) 685-696. |
| [12] | Y.L.N. Murthy, B. Govindh, B.S. Diwakar, K. Nagalakshmi, K.V.R. Rao, Synthesis and bioevaluation of Schiff and Mannich bases of isatin derivatives with 4-amino-5-benzyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, Med. Chem. Res. 21 (2012) 3104-3110. |
| [13] | S.N. Pandey, D. Sriram, G. Nath, E. DeClercq, Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4'-chlorophenyl)thiazol-2-yl] thiosemicarbazide, Eur. J. Pharm. Sci. 9 (1999) 25-31. |
| [14] | A.D. Kulkarni, S.A. Patil, P.S. Badami, SNO donor Schiff bases and their Co(Ⅱ), Ni(Ⅱ) and Cu(Ⅱ) complexes: synthesis, characterization, electrochemical and antimicrobial studies, J. Sulfur Chem. 30 (2009) 145-159. |
| [15] | M.C. Rodríguez-Argüelles, R. Cao, A.M. García-Deibe, et al., Antibacterial and antifungal activity of metal(Ⅱ) complexes of acylhydrazones of 3-isatin and 3-(N-methyl)isatin, Polyhedron 28 (2009) 2187-2195. |
| [16] | K. Haj Mohammad Ebrahim Tehrani, F. Kobarfard, P. Azerang, et al., Synthesis and antimycobacterial activity of symmetric thiocarbohydrazone derivatives against Mycobacterium bovis BCG, Iran. J. Pharm. Res. 12 (2013) 331-346. |
| [17] | K. Haj Mohammad Ebrahim Tehrani, S. Sardari, V. Mashayekhi, et al., One pot synthesis and biological activity evaluation of novel Schiff bases derived from 2-hydrazinyl-1,3,4-thiadiazole, Chem. Pharm. Bull. 61 (2013) 160-166. |
| [18] | V. Mashayekhi, K. Haj Mohammad Ebrahim Tehrani, S. Amidi, F. Kobarfard, Synthesis of novel indole hydrazone derivatives and evaluation of their antiplatelet aggregation activity, Chem. Pharm. Bull. 61 (2013) 144-150. |
| [19] | V. Mashayekhi, K. Haj Mohammad Ebrahim Tehrani, P. Azerang, S. Sardari, F. Kobarfard, Synthesis, antimycobacterial and anticancer activity of novel indolebased thiosemicarbazones, Arch. Pharm. Res. (2013), http://dx.doi.org/10.1007/s12272-013-0242-z. |
| [20] | K. Haj Mohammad Ebrahim Tehrani, V. Mashayekhi, P. Azerang, et al., Synthesis and antimycobacterial activity of novel thiadiazolylhydrazones of 1-substituted indole-3-carboxaldehydes, Chem. Biol. Drug Des. 83 (2014) 224-236. |
| [21] | J. Handzlik, E. Szymańska, J. Chevalier, et al., Amine-alkyl derivatives of hydantoin: new tool to combat resistant bacteria, Eur. J. Med. Chem. 46 (2011) 5807-5816. |
| [22] | E. Szymańska, K. Kieć-Kononowicz, Antimycobacterial activity of 5-arylidene aromatic derivatives of hydantoin, Ⅱ Farmaco 57 (2002) 355-362. |
| [23] | R.P. Bhole, K.P. Bhusari, Synthesis and 3D-QSAR of p-hydroxybenzohydrazide derivatives with antimicrobial activity against multidrug-resistant Staphylococcus aureus, J. Korean Chem. Soc. 54 (2010) 77-87. |
| [24] | K.N. Myangar, J.P. Raval, Design, synthesis, and in vitro antimicrobial activities of novel azetidinyl-3-quinazolin-4-one hybrids, Med. Chem. Res. 21 (2012) 2762-2771. |
| [25] | Z.J. Liang, D.Y. Zhang, J. Ai, et al., Identification and synthesis of N'-(2-oxoindolin-3-ylidene)hydrazide derivatives against c-Met kinase, Bioorg. Med. Chem. Lett. 21 (2011) 3749-3754. |
| [26] | K. Debnath, S. Pathak, A. Pramanik, Facile synthesis of ninhydrin and isatin based hydrazones in water using PEG-OSO3H as a highly efficient and homogeneous polymeric acid-surfactant combined catalyst, Tetrahedron Lett. 54 (2013) 4110-4115. |
| [27] | L. Tripathi, R. Singh, J.P. Stables, Design & synthesis of N'-[substituted] pyridine-4-carbohydrazides as potential anticonvulsant agents, Eur. J. Med. Chem. 46 (2011) 509-518. |
| [28] | T. Aboul-Fadl, H.A. Abdel-Aziz, M.K. Abdel-Hamid, et al., Schiff bases of indoline-2,3-dione: potential novel inhibitors of mycobacterium tuberculosis (Mtb) DNA gyrase, Molecules 16 (2011) 7864-7879. |
| [29] | M. Hassan, Y. Javadzadeh, F. Lotfipour, R. Badomchi, Determination of comparative minimum inhibitory concentration (MIC) of bacteriocins produced by enterococci for selected isolates of multi-antibiotic resistant Enterococcus spp., Adv. Pharm. Bull. 1 (2011) 75-79. |
| [30] | C.A. Lipinski, F. Lombardo, B.W. Dominy, P.J. Feeney, Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv. Drug Deliv. Rev. 46 (2001) 3-26. |
 2016, Vol.27
2016, Vol.27