b Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China
"Veratrum nigrum has antagonistic features against Panax ginseng" which belongs to one of the eighteen incompatible medicaments of traditional Chinese medicine. But in recent years,based on intense ongoing studies,some medical experts believe that the proper use of an incompatible drug could produce a stronger effect for the treatment of diseases. In addition,several experiments have revealed that the toxicity of an incompatible drug was related to the absolute dose and relative dose with another drug [1]. Recently,the relevant studies on compatibility of Veratrum nigrum and Panax ginseng were mainly in the areas of chemical changes,toxicity effect on HepG2 cells,enzyme activity effect on CYP1A and relevant animal experiments [2, 3, 4, 5, 6]. However,what is it likely that the Veratrum alkaloids absorbed through the human intestine before and after compatibility with Panax ginseng? In this study,the transport of Veratrum alkaloids influenced by Panax ginseng was investigated through the Caco- 2 cells,which can imitate the transport in vitro. It is particularly important to clarify the interaction mechanism between the drugs from the perspective of intestinal absorption.
Currently,the Caco-2 cells model is shown to be an effective means to study drug absorption [7, 8]. The Caco-2 cells are derived from human colon cancer cells,and can be cultured in vitro to be epithelial differentiation and form tight junctions. The cells can differentiate to cover the villus surfaces (apical side,AP,intestinal cavity side) and substrate surfaces (basolateral side,BL,intestinal wall side). Fortunately,their structure and biochemical properties are similar to the human intestinal epithelial cells with the same cell polarity and tight junctions and the cells can express the same various transporters and metabolic enzymes as the intestinal epithelial cells. The Caco-2 cells have been recognized as effective tools to be applied in the studies of drug uptake,metabolism,excretion and transport.
To investigate the effect on Veratrum alkaloids,a HPLCmethod was established for the determination of veratramine,and a UPLC/Q-TOF MS method was developed for qualitative investigation of the Veratrum alkaloids [9, 10]. According to the reports in the literature,the UPLC-ESI-MS method was widely used in the analysis of biological samples [11, 12]. In this study,the new UPLC-ESI-MS method and IS method were validated so that the content changes of several Veratrum alkaloids could be detected semi-quantitatively at the same time. The UPLC-ESI-MS method was used in the analysis of biological samples to determine the uptake of the Veratrum alkaloids compatible with Panax ginseng at different proportions through the Caco-2 cell monolayer model. And this study provided a theoretical basis for the further study of compatibility of traditional Chinese medicine from the perspective of intestinal absorption.
2. Experimental 2.1. MaterialsCaco-2 human colon carcinoma cell line was obtained from Shanghai Cell Bank of Chinese Academy of Sciences. Panax ginseng was taken from the ginseng field of Jilin Fusong; Veratrum nigrum was supported by Anhui Fengyuan Tongling Chinese herbal medicine company. Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Corning Corporation (USA); Heat-inactivated fetal bovine serum (FBS) was purchased from Biological Industries Israel Beit Haemek Ltd. (USA); MTT,Trypsin and Penicillin were purchased from Dingguo Corporation (Beijing,China); DMSO was purchased from Xilong Chemical Corporation (Guangdong,China); streptomycin was purchased from Genview Corporation (USA); Berberine was purchased from National Institutes for Food and Drug Control (Beijing,China); methanol and acetonitrile were purchased from Fisher Scientific (America). Milli-Q water (Millipore Inc.,USA) was used in the study; all other chemicals were of analytical grade or better. The MCO-175 CO2 incubator was purchased from SANYO (Japan); high speed refrigerated centrifuge was purchased from Eppendorf (Germany); enzyme mark instrument was purchased from TECAN (Australia); 96-well culture plate was purchased from Thermo (USA); transwell cell culture plate (1.12 cm surface,0.4 mm pore size,12 mm diameter) was purchased from Corning Costar Corporation (USA).
2.2. Sample preparationVeratrum nigrum (20 g),or Veratrum nigrum,with different proportions of Panax ginseng (20 g) was extracted twice with boiling water,one hour each time,and the solutions were filtered through centrifugation. Then,the filtrates were precipitated by 70% alcohol to remove the polysaccharide and concentrated to 1 g/mL. The single extract,Veratrum nigrum,and the mixed extract,Veratrum nigrum with Panax ginseng,were freeze dried and subsequently,these dried extracts were dissolved in DMEM,and then,the generated solutions were used to obtain the working standard solutions used in the study of MTT.
However,in the study of transport,the single Veratrum nigrum extract was dissolved in HBSS to obtain the working solutions. The final concentrations of the samples of the transport study were 2 mg/mL,4 mg/mL,8 mg/mL,respectively. The mixed extracts were also dissolved in HBSS to obtain the final concentrations of Veratrum nigrum in the transport samples at 2 mg/mL. All the cell transport samples were freeze dried for further testing.
Prior to detection by UPLC-ESI-MS,berberine (IS,200 μg/L,dissolved in methanol) was added to the Caco-2 cell transport sample (v/v = 50/50) to 100 μL.
2.3. Cell cultureThe Caco-2 cells were cultured in DMEM high-glucose medium containing 10% fetal bovine serum,as well as 100 U/mL penicillin and streptomycin. The cells were grown at 37 ℃ with 5% CO2. 2.3.1. Cytotoxicities of the single Veratrum nigrum extract and the mixed extract with different proportions of Panax ginseng on Caco-2cells
The Caco-2 cells were seeded in 96-well culture plates at a density of 2× 104 cells per well and were grown at 37 ℃ with 5% CO2 for 48 h. Then,the cells were treated with a series of working solutions for 2.5 h. The Veratrum nigrum extracts,or the mixed extracts with different proportions of Panax ginseng,were removed and MTT was added,100 μL per well (1 mg/mL) for four hours. Then,MTT was removed and DMSO was added,100 mL per well. When all the blue crystallized material generated through above procedure was dissolved by shaking for 10-15 min,the absorbance of the plate was measured at 570 nm.
2.3.2. Transport studiesFor transport studies,firstly,the Caco-2 cells were seeded in transwell polycarbonate insert filters at a density of 1 × 105 cells per well and were grown at 37 ℃ with 5% CO2. After 21 days of cell culture,the cells can fusion to form a continuous and complete single-cell layer. After morphological observation and trans epithelial electrical resistance (TEER) testing,the Caco-2 cell monolayer model was determined to form successfully.
Prior to the experiments,the cell monolayers were rinsed twice using Hank’s balanced salt solution (HBSS) to remove the DMEM medium completely,and the cells were then incubated with HBSS at 37 ℃ for 30 min. Then,the cell monolayers were treated with transport medium containing Veratrum nigrum extract,or mixed extract of Veratrum nigrum and Panax ginseng,respectively,in the AP or BL side.
When the transport from AP to BL was studied,for example,0.5 mL transport medium containing herbal extract was added to the AP side,and the BL side was treated with 1.5 mL HBSS. Then,a 100 mL of the incubation solution was absorbed at times of 20,40,60,90 and 120 min,respectively,from the BL side,and supplemented with the same volume of 37 ℃ HBSS to the BL side each time. At last,terminate the transportation with 4 ℃ HBSS. All the samples were freeze dried for further analysis.
When the transport from AP to BL was studied,for example,0.5 mL transport medium containing herbal extract was added to the AP side,and the BL side was treated with 1.5 mL HBSS. Then,a 100 mL of the incubation solution was absorbed at times of 20,40,60,90 and 120 min,respectively,from the BL side,and supplemented with the same volume of 37 ℃ HBSS to the BL side each time. At last,terminate the transportation with 4 ℃ HBSS. All the samples were freeze dried for further analysis.
2.4. UPLC-ESI-MS methodThe UPLC-ESI-MS was used to determine the level of compounds in the transport samples. The conditions of UPLC for analysis were as follows: system: Waters Acela U-HPLC with Acela temperature controlled auto sampler and column oven and Acela 1250 ultra-high pressure liquid pump; column: ACQUITY UPLC BEH C18,1.7 mm,2.1 × 50 mm (Waters,USA); mobile phase A,acetonitrile; mobile phase B,0.1% (v/v) formic acid aqueous solution; gradient,0-1 min,10% A,1-3 min,10%-20% A,3-5 min,20%-25% A,5-15 min,25%-30% A,15-20 min,30%-90% A; flow rate,0.3 mL/min; injection volume,10 μL.
The specific conditions of the MS for the analysis were as follows: LTQ XL ion trap mass spectrometer with electrospray ionization (ESI) source (Thermo,USA). Positive ion mode,mass scan ranged from m/z 300 to 1000,spray voltage was 5 kV,the metal capillary temperature was 250 ℃,metal capillary voltage was 40 V,sheath gas flow rate of 50 L/h,auxiliary gas flow rate of 5 L/h,lens voltage was 110 V.
2.5. Data analysisThe apparent permeability coefficient (Papp) was calculated as follows [13]:

Meanwhile,the recovery rate of each compound was calculated as follows: recovery (%) = the quantity of a compound in donor and receiver chamber at the end of the experiment/initial quantity of this compound in donor chambere × 100% [14].
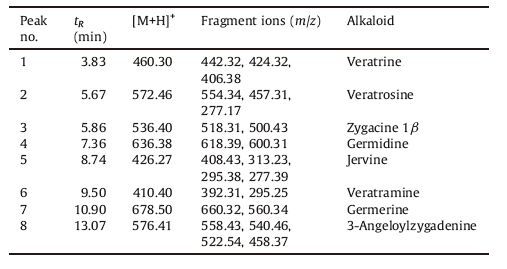
3. Results and discussion 3.1. UPLC-ESI-MS method for the separation and identification of the main alkaloidsThe transport samples were analyzed by UPLC-ESI-MS method and the total ion chromatogram (TIC) is presented in Fig. 1. The retention time and the separation fractions of the eight Veratrum alkaloids are presented in Table 1.
The eight alkaloids below were separated and identified through the method of UPLC-ESI-MS based on matching their retention times,detection of the [M+H]+ and fragment ions obtained through the tandem mass spectrometry with the related literature. The eight alkaloids were veratrine,veratrosine,zygacine 1β,germidine,jervine,veratramine,germerine and 3-angeloylzygadenine,respectively [15].
The internal standard (IS) method is used for quantitative research of alkaloids. The IS method is a more accurate quantitative method in chromatography,especially when there are no standard controls. The IS method involves using a certain amount of the pure substance as a reference standard which is added to a certain amount of analyte in the sample. Then,the samples containing the IS are analyzed by chromatography,and peak areas of the analytes and IS component in the total ion chromatogram are acquired and a relative correction factor can be calculated according to the formula. The components of the analytes in the sample can then be calculated,and the other various indicators,such as Papp and Er,calculated according to the relative formulas.
The calibration curves were constructed on the basis of the peak area ratios of the analytes to internal standard versus the concentrations of the analytes. The correlation coefficient was higher than 0.98. The RSDs of the retention times and the peak areas of IS and the analytes were all below 0.73% and 6.32%,which illustrates that the stability of the sample solutions and the accuracy of the method were all acceptable.

|
Download:
|
| Fig. 1.The total ion chromatogram of Veratrum alkaloids. | |
|
|
Table 1 The retention time and identification of the Veratrum alkaloids. |
To study the effect of time on the uptake of Veratrum alkaloids,the transports of AP to BL and BL to AP of the Veratrum alkaloids at 8 mg/mL were investigated over a period of 2 h. The transport medium containing 8 mg/mL Veratrum nigrum extract was added to the AP side or BL side. Samples were absorbed individually at 20,40,60,90 and 120 min and determined by UPLC-ESI-MS. A point is presented as the mean ± RSD of three determinations. As shown in Fig. 2,it is confirmed that the transport of the alkaloids in both directions increased gradually with time. The results show that time was an important factor in the transport of Veratrum alkaloids,such as,both absorption and excretion of Veratrum alkaloid were timedependent. However,the transport of each alkaloid influenced by time is different. Jervine,3-angeloylzgadenine and veratramine are more vulnerable to the impact of time than the other five alkaloids from both AP to BL and BL to AP.

|
Download:
|
| Fig. 2.Effect of time on the transport of the Veratrum alkaloids: (a) AP to BL, (b) BL to AP. | |

|
Download:
|
| Fig. 3.Effect of concentration on the transport of Veratrum alkaloids: (a) AP to BL, (b) BL to AP. | |
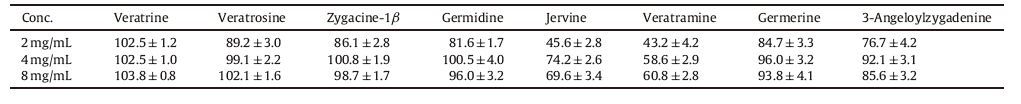
To study the effect of concentration on the uptake of the Veratrum alkaloids,the transports of AP to BL and BL to AP of the Veratrum alkaloids at three different concentrations were observed over a period of 2 h. At different concentrations,2 mg/mL,4 mg/mL,8 mg/mL,of Veratrum extract were added to the AP side,or BL side. Samples were absorbed at 20,40,60,90 and 120 min,respectively and determined by UPLC-ESI-MS. The Papp values of the eight Veratrum alkaloids at 120 min are shown in bar graphs (Fig. 3a). From this figure,it can be seen that the transports of the alkaloids in the AP to BL direction increased gradually with the increases of the concentrations ranging from 2 mg/mL to 4 mg/mL,but when the concentration increased to 8 mg/mL,the transport was not shown to increase further. Meanwhile,the transports of the alkaloids in BL to AP direction did not change with the increases of the concentrations as showed in Fig. 3b. This means that the concentration was important for the transport of the Veratrum alkaloids from AP to BL,but not important for the excretion. And germidine and germenine are more vulnerable to the impact of their concentrations,while veratrosine was less affected by changes in concentration. This may be related to their different levels of fat-solubility and dissociation.
3.4. Recovery rates of Veratrum alkaloids from AP to BL in the Caco-2cellsTo study the recovery rates of Veratrum alkaloids across the Caco-2 cell,three different concentrations of Veratrum nigrum extract were added to the AP side. During the 120 min of the transport,100 mL incubation solution were absorbed from the AP side and BL side,respectively,at different time points. And the initial transport medium containing herbs extract and the incubation solution from the AP side and BL side at 120 min were all analyzed by UPLC-ESI-MS. The content of each alkaloid is calculated according to their concentrations measured by the IS method and volume of incubation solution from the AP side and BL side. Then,according to the formula in "2.5 Data analysis" of this article,the recovery of each alkaloid was calculated.
Jervine and veratramine both show lower recovery rates than the other 6 Veratrum alkaloids as shown in Table 2. It was predicted that jervine and veratramine may accumulate in the Caco-2 cells,or metabolize by the enzyme systems in Caco-2 cells when absorption from AP to BL [14]. Jervine and veratramine,or their metabolites may be released from the Caco-2 cells and display their corresponding affects subsequently. However,further studies must be carried out to confirm this hypothesis.
|
|
Table 2 Recovery rates (mean ± SD, n = 3) of the eight Veratrum alkaloids. |
Transports of the Veratrum alkaloids with different proportions of Panax ginseng were observed to resolve whether the uptake of Veratrum alkaloids is influenced by Panax ginseng in human intestine. Subsequently,two cases were studied involving the single Veratrum nigrum extract,and another one at different compatibility proportions of 1:1,2:1,10:3,10:1,20:1 of Panax ginseng and Veratrum nigrum. As shown in Fig. 4a,the absorptions of all the eight Veratrum alkaloids increased compared to the single Veratrum nigrum extract. However,the excretions of the Veratrum alkaloids presented different varying rules (Fig. 4b).

|
Download:
|
| Fig. 4.The transports of the Veratrum alkaloids at different proportions of Panax ginseng: (a) AP to BL, (b) BL to AP. | |
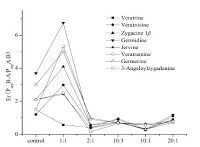
Drug efflux pump action is the main reason of low drug bioavailability. With the presence of the transport protein,such as P-glycoprotein,the actual extent of absorption of drugs will decline. The efflux ratio (Er),which is a ratio of PappB-A and PappA-B,can intuitively reflect the actual absorption through the Caco-2 cells in the presence of the excretion [16]. From Fig. 5 we can see that when the ratio for the compatibility of Panax ginseng and Veratrum nigrum is 1:1,the efflux ratio of the Veratrum alkaloids,except for veratrine,increased compared to the single Veratrum nigrum extract. When the compatibility proportions were 2:1,10:3,10:1 and 20:1,respectively,the absorptions of the Veratrum alkaloids were superior to the effluxes,so that the bioavailability was raised. That is to say,the excretion was greater than the absorption,thus the bioavailability of the Veratrum alkaloids was reduced. While on the other compatibility proportions,the efflux ratio of the Veratrum alkaloids decreased compared to the single Veratrum nigrum extract. But the absorptions were greater than the excretions; therefore,the bioavailability of the Veratrum alkaloids is improved as shown in Fig. 5.
|
Download:
|
| Fig. 5.The efflux ratio (PappB-A/PappA-B) of the Veratrum alkaloids. | |
According to the literature reports,the pharmacological effects of the Veratrum alkaloids were mainly reflected on the cardiovascular system,and the Veratrum alkaloids revealed some bioactivities,such as antifungal,antiviral,anti-schistosomiasis,anti-tuberculosis and emetic effects [17]. Veratramine was one of the main active constituents of the Veratrum alkaloid with the effects of depressing blood pressure [18]. Germidine had the effect of anti-experimental arteriovenous thrombus,which related to its functions of inhibiting platelet aggregation; reducing blood viscosity and as anticoagulant [19]. Veratramine and jervine had inhibitory effects on Bacillus subtitles,Escherichia coli,Aspergillus candidus and Staphylococcus aureus [20]. Veratramine,jervine and veratrosine had the inhibition effects individually on A549,PANC-1,SW1990,and NCI-H 249 [21]. Veratrosine could inhibit cardio acceleration induced by epinephrine and norepinephrine [22]. Jervine and veratramine could inhibit the migration and proliferation of prostate cancer [23]. Veratramine and germerine could influence angiogenesis [24]. If the hypothesis that the pharmacological effects are related to the absorption in human intestinal is true,then the effects of Veratrum alkaloids could be improved when combined with Panax ginseng in suited proportions.
It is necessary to point out that the Veratrum alkaloids have certain toxic effects on the nervous,respiratory,cardiovascular and digestive systems [25]. Therefore,the absorption increment of the Veratrum alkaloids has duplicities of pharmacodynamic actions and toxicity effects. Thus,further studies should be initiated on proving the safety of the compatibility of Veratrum nigrum with Panax ginseng.
4. ConclusionThe UPLC-ESI-MS method was widely used in the analysis of biological samples. This method was successfully applied to the analysis of trace amounts of drugs in biological sample,which exhibited good precision,repeatability and accuracy. In this study,a rapid UPLC-ESI-MS method was developed for the separation and identification of the Veratrum alkaloids and detection of their transport,through the Caco-2 cell model in vitro. The results suggest that the flux of Veratrum alkaloids were time and concentration dependent,and the absorption of all eight Veratrum alkaloids increased compared to the single Veratrum nigrum extract. However,the excretions of the Veratrum alkaloids presented different varying rules. This provided a new method to study the Veratrum alkaloids in biological samples,which had important pharmacological effects as well as certain toxicity effects on human body. Additionally,this study provided a theoretical basis for the further study of compatibility of traditional Chinese medicine from the perspective of intestinal absorption.
5. AcknowledgmentsThis research was supported by the National Natural Science Foundation of China (Nos. 81073040,81274046,81173507),the National Basic Research Program of China (National 973 Program) (Nos. 2011CB505300,2011CB505305) and Jilin Province Science and Technology Agency-funded projects (No. 20150414040GH).
| [1] | J. Park, Y.D. Jeon, H.L. Kim, et al., Interaction of Veratrum nigrum with Panax ginseng against obesity: a sang-ban relationship, Evid. Based Complement. Altern. Med. 2013 (2013) 732126. |
| [2] | X. Zhang, F.R. Song, L.S. Wang, et al., Studies on the content variation of chemical constituents during the combination of ginseng with Veratrum nigrum by ESI-MS and HPLC-ESI-MS, Acta Chim. Sin. 65 (2007) 829-833. |
| [3] | Y.L. Lu, A.H. Sun, Y. Gao, Y. Jiang, In vitro assessment of increasing cytotoxicity of Veratrum nigrum induced by Panax ginseng, Mil. Med. Sci. 38 (2014) 285-289. |
| [4] | C.Y. Wang, X. Ye, Y.G. Wang, et al., Modulation of the enzyme activities of cytochrome P450 1A in Rat Livers by Panax gingseng and Coadministration with Veratrum nigrum, Pharm. J. Chin. PLA 26 (2010) 104-106. |
| [5] | Y.G. Wang, Y. Gao, B.X. Xin, et al., Modulation of the activities and mRNA expression of cytochrome P450 isoenzymes in rat liver by Panax gingseng and coadministration with Veratrum nigrum, Chin. J. Chin. Mat. Med. 29 (2004) 366-370. |
| [6] | Y. Lin, E.X. Shang, Y. Xu, et al., Influence of Veratrum nigrum to Panax gingseng's Anti-fatigue based on uniform design, Chin. J. Exp. Tradit. Med. Formulae 20 (2014) 124-128. |
| [7] | M.A. Júnior, A.C. de Faria, S. Velozo Eda, et al., Determination of fexofenadine in Hank's balanced salt solution by high-performance liquid chromatography with ultraviolet detection: application to Caco-2 cell permeability studies, Biomed. Chromatogr. 29 (2015) 537-544. |
| [8] | S. Sun, H. Zhang, F.F. Sun, et al., Intestinal transport of sophocarpine across the Caco-2 cell monolayer model and quantification by LC/MS, Biomed. Chromatogr. 28 (2014) 885-890. |
| [9] | Y. Cong, J.H. Wang, Y. Wang, Determination of veratramine in crude dried and processed roots and rhizomes of Veratrum nigrum L. by HPLC, J. Henan Univ. Med. Sci. 27 (2008) 14-16. |
| [10] | Y.G. Wang, C. Wang, Q.D. Liang, et al., Analysis of chemical composition in combination of Veratrum nigrum and radix ginseng by UPLC/Q-TOFMS with multivariate statistical analysis, Sci. Sin. Vitae 41 (2011) 925-932. |
| [11] | J.S. Ma, S.H. Wang, M.L. Zhang, et al., Simultaneous determination of bupropion, metroprolol, midazolam, phenacetin, omeprazole and tolbutamide in rat plasma by UPLC-MS/MS and its application to cytochrome P450 activity study in rats, Biomed. Chromatogr. 29 (2015) 1203-1212. |
| [12] | X.B. Cui, X.C. Qian, P. Huang, et al., Simultaneous determination of ten flavonoids of crude and wine-processed Radix Scutellariae aqueous extracts in rat plasma by UPLC-ESI-MS/MS and its application to a comparative pharmacokinetic study, Biomed. Chromatogr. 29 (2014) 1112-1123. |
| [13] | P. Artursson, Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells, J. Pharm. Sci. 79 (1990) 476-482. |
| [14] | J. Yu, N. Li, P. Lin, et al., Intestinal transportations of main chemical compositions of Polygoni multiflori Radix in Caco-2 cell model, Evid. Based Complement. Alternat. Med. 2014 (2014) 483641. |
| [15] | J.M. Sun, The HPLC-MS method to study the substance foundation of opposite and complementary of Panax ginseng and Veratrum nigrum in Tongdingsan, J. Changchun Univ. Tradit. Chin. Med. 5 (2012) 909-910. |
| [16] | H. Kan, W.Y. Jiang, R. Ding, et al., Studies of the intestinal absorption of the alkaloids in the Wu-tou decoction combined with different incompatible medicinal herbs in a Caco-2 cell culture system using UPLC-MS/MS, Chin. Chem. Lett. 26 (2015) 590-594. |
| [17] | J.T. Han, Pharmacological action and clinical application of Veratrum nigrum, Mod. Health 27 (2011). |
| [18] | Q. Wang, W. Li, M. Yang, et al., Experimental study of the antihypertensive effect of Vatramine, Chin. J. Gerontol. 16 (2011). |
| [19] | L. Lv, P. Pan, G.Z. Han, et al., Study on anti-arterial thrombosis effect of germidine, an alkaloid isolated from Veratrum nigrum L. var ussuriense Nakai, J. Dalian Med. Univ. 33 (2011) 317-320. |
| [20] | B. Xue, Study on extraction, purification and antimicrobial activity of alkaloid from Veratrum nigrum, (Master thesis), Nanchang University, 2013. |
| [21] | J. Tang, H.L. Li, Y.H. Shen, et al., Antitumor activity of extracts and compounds from the rhizomes of Veratrum dahuricum, Phytother. Res. 22 (2008) 1093-1096. |
| [22] | O. Krayer, Studies on Veratrum alkaloids: the inhibition by Veratrosine of the cardioaccelerator action of epinephrine and of norepinephrine, J. Pharmacol. Exp. Ther. 97 (1949) 256-265. |
| [23] | M.A. Khanfar, K.E. Sayed, The Veratrum alkaloids jervine, veratramine, and their analogues as prostate cancer migration and proliferation inhibitors: biological evaluation and pharmacophore modeling, Med. Chem. Res. 22 (2013) 4775-4786. |
| [24] | W. Poethke, Mechanism of vasomotor action of Veratrum alkaloids: extravagal sites of action of veriloid, protoveratrine, germitrine, neogermitrine, germerine, veratridine and veratramine, J. Pharmacol. Exp. Ther. 113 (1955) 100-114. |
| [25] | Y. Zhao, Progress in pharmacological and toxicological study of Veratrum alkaloid, Toxicology 04 (2007). |
 2016, Vol.27
2016, Vol.27