Fluorescence probe has become a widely used and important tool for monitoring metal ion concentration in biological samples. Most sensors reported have a metal chelating site linked to a fluorophore,and the metal binding causes a change in fluorescence intensity [1, 2, 3, 4, 5, 6]. The development of synthetic receptors enabled the recognition of important metal ions,especially transitionmetal ions,in biologically and environmentally relevant samples. This has attracted widespread interest from biologists,chemists,environmentalists and clinical biochemists in recent years [7, 8, 9,10,11,12]. The metal ions,like platinum,mercury,zinc and palladium have environmental and biological importance.
Palladium belongs to the platinum-group elements (PGEs; consist of Pt,Pd,Ru,Rh,Ir and Os,). It is widely used in various materials such as catalysts,dental crowns,jewelry and fuel cells [13, 14, 15]. Pd-catalyzed reactions such as the Heck,Sonogashira,Buchwald-Hartwig and Suzuki-Miyaura reactions represent powerful transformations for the synthesis of complex molecules,which played an important role in pharmaceuticals [16, 17, 18, 19, 20, 21, 22, 23]. However,their fruitful and frequent use can also result in the contamination of soil and water systems [23] and therefore could cause health hazards [24, 25, 26]. The restrictions of government on the residual heavy metals in products are very critical. Therefore,palladium detection both in living systems and in environment has attracted tremendous attention.
Detecting the content of palladium was usually carried out by atomic absorption/emission spectroscopy,ion-coupled plasma emission-mass spectroscopy,solid-phase microextraction/high performance liquid chromatography,and X-ray fluorescence spectroscopy [27, 28]. Although these conventional methods provide an extremely sensitive and rapid analysis,they need sophisticated sample-pretreatment procedures,complicated instrumentation and rigorous experimental conditions.
In recent years,the fluorescence method has been developed for palladium analysis and the colorimetric technique has frequently been applied [29, 30, 31]. For example,palladium could be detected by fluorescent ligands via fluorescence quenching [32, 33, 34, 35, 36, 37, 38]. Schwarze et al. designed the first chemical sensors for Pd2+ detection through increasing fluorescence [39].
Rhodamines are dyes widely employed in the study of complicated biological systems as fluorescence probes due to their high fluorescence quantum yields,high absorption coefficients,long-wavelength emissions and absorptions [40]. Owing to the spirolactam scaffold in rhodamines,which undergoes a conformational transformation from the spirolactam (nonfluorescent and colorless) to an open-ring structure (fluorescent and colored),they have been widely applied in the design of chemical sensors in recent years,and these new designed chemosensors have been reviewed recently [41, 42, 43, 44]. In this spirocyclic form,the sensing event occurs in its five-membered lactam moiety. Obviously,these properties still present abundant opportunities for the design of new fluorescent probes.
Our research work encompasses the design,synthesis,spectroscopy and biological applications of fluorescent chemosensors for selective sensing of Pd2+. Here we are reporting a novel rhodamine based chemosensor T1,which was prepared by a threestep synthesis using inexpensive materials. Particularly,the chemosensor showed highly selective and sensitive fluorescence "turn-on" behaviors toward Pd2+ followed the remarkable color changes from colorless to pink,which can be used for "naked eyes" detection. Moreover,the probe can give highly selective spectroscopic responses to Pd2+ over other metal ions in living cells,which showed T1 can penetrate cell membranes and react with Pd2+ within living cells.
2. Experimental2.1. Materials
All the solvents and reagents were of analytic grade and they were used without further purification unless for special needs. The T1 was dissolved in DMF-MeOH in concentration of 1 mmol/L as stock solution. And then quantificational T1 was used for different testing systems. We used the HITACHI F-4500 fluorescence spectrophotometer to measure the fluorescence spectra. And the absorbance spectra measurements were also performed on a Shimadzu UV-1700 spectrophotometer. IR spectra were collected on a Bruker Tensor 27 spectrometer. NMR spectra were recorded on a Varian INOVA -400 MHz spectrometer (at 100 MHz for 13C NMR and 400 MHz for 1H NMR). A Bruker micro TOF-Q II ESI-TOF LC/MS/MS Spectroscopy was used to perform mass spectra. And the living cells imaging was performed on an Olympus FV1000 confocal microscope. We set the excitation wavelength as 540 nm. All reagents used for synthesis were of analytical-reagent grade and commercially available from Aldrich. Thin-layer chromatography (TLC) and column flash chromatography were performed using silica gel GF254 and Merck silica gel (250-400 mesh ASTM),respectively. And the twice-distilled water was used throughout the experiment.
2.2. SynthesisSynthesis of compound 2: We synthesized the compound 2 from Rhodamine B using the procedures published in literature [45, 46, 47, 48].
Synthesis of compound 3: In a 100 mL flask,an excess of isophthalaldehyde (0.268 g,0.002 mol) was dissolved in 20 mL of methanol,cooled in a cold water bath. Then Rhodamine hydrazide (0.461 g,0.001 mol) was dissolved in 30 mL of methanol and added dropwise to the above solution with vigorous stirring,and the stirred mixture was allowed to stand in the cold water bath for about 4 h,then the solvent was removed under reduced pressure,the crude was purified by silica gel column chromatography to give 3 (white powder) in 78.8% yield. MALDI-TOF MS calcd. for (C36H36N4O3) m/z = 573.2860 (M+ + 1),Found: 573.2802. 1H NMR (400 MHz,CDCl3): δ 1.13 (t,12H,J = 8 Hz),3.24-3.29 (m,8H),6.41-6.43 (m,2H),6.63-6.67 (m,4H),7.11 (d,1H,J = 8 Hz),7.37 (t,1H,J = 8 Hz),7.53-7.57 (m,3H),7.73 (d,1H,J = 8 Hz),7.98 (d,1H,J = 8 Hz),8.16 (d,1H,J = 8 Hz),9.18 (s,1H),9.64 (s,1H). 13C NMR (100 MHz,CDCl3): δ 192.2,165.2,153.1,151.9,149.0,144.8,136.6,136.4,133.6,132.8,129.9,129.4,129.0,128.9,128.4,127.9,123.9,123.5,
108.0,105.8,97.9,44.3,12.6.
Synthesis of Compound 4: Compound 3 (0.572 g,0.001 mol) was dissolved in 20 mL of methanol. Then a solution of 2- aminophenol (1.2 g,a little excess) in methanol (20 mL) was added and the mixture was stirred for 2 h at 60 ℃. Then the precipitates were collected and washed 3 times with 10 mL of cold ethanol. After drying under reduced pressure,the crude product was purified by recrystallization in CH3CN/H2O to give compound 4 (white solid) in 82.6% yield. IR (KBr/cm-1): υ 3444,2967,2925,1694,1615,1=0,1512,1460,1426,1356,1307,1270,1223,1120,1072,1011,980,946,873,820,786,7=
,688. MALDI-TOF MS calcd. for (C42H41N5O3) m/z = 663.3282,Found: 663.3298. 1 H NMR (400 MHz,CDCl3) δ: 1.15 (t,12H,J = 8 Hz),3.30-3.35 (m,8H),6.23-6.27 (m,2H),6.49-6.54 (m,4H),6.92 (t,1H,J = 4 Hz),7.02 (d,1H,J = 4 Hz),7.12-7.17 (m,2H),7.28-7.31 (m,1H),7.37-7.49 (m,3H),7.77-7.82 (m,2H),7.97-8.03 (m,2H),8.64 (s,1H),8.82 (s,1H),9.96 (s,1H). 13C NMR (100 MHz,CDCl3): δ12.6,44.1,66.2,97.9,105.8,115.1,120.1,123.9,127.9,129.0,135.9,145.9,151.5,152.4,165.0. (Scheme 1).
|
Download:
|
| Scheme 1.Synthesis of 4. | |
The 10 μmol/L stock solution of probe T1 was prepared in methanol and water (4/6,v/v). The solutions of various testing cation species were prepared from CaCl2,AgNO3,MgCl2,CoCl2·6H2O,CdCl2,ZnCl2,CuCl2·2H2O,MnSO4·H2O,HgCl2,NiCl2·6H2O,Pb(NO3)2,CrCl3·6H2O,PdCl2,BaCl2·2H2O,NaCl,AlCl3·6H2O and FeCl3·6H2O in the doubly distilled water. Before spectroscopic measurements,the corresponding solutions of 4 were freshly prepared by diluting the high concentration stock solution. All the measurements were made according to the procedures as follows. Placing 1 mL of the probe solution and an appropriate aliquot of each metal stock into a 10 mL glass tube,and diluting the solution to 10 mL with methanol-water (4/6,v/v). The absorbance was at =0 nmand the fluorescence emission appeared at 583 nm. Both the excitation and emission wavelength band passes were set as 5.0 nm and the excitation wavelength was set at 540 nm.
2.4. Cell culture and fluorescence imagingThe human cancer cell line HepG2 (liver cells) were cultured in RPMI 1640 replenished with 10% FBS. Before the experiments,cells were preprocessed with probe T1 (10 μmol/L) for 1 h at 37 ℃ in humidified air and 5% CO2,washed three times with PBS then imaged. After incubation with Pd2+ (10 μmol/L) for another 1.5 h at 37 ℃,cells were washed 3 times with PBS to remove remaining Pd2+ and then imaged. Confocal fluorescence imaging was carried out using an Olympus FluoView FV1000 laser scanning microscope with a 40× objective lens. Excitation of 1-loaded cells at 540 nm was performed with a solid laser and emission was collected at =0-750 nm.
3. Results and discussion3.1. Spectroscopic properties
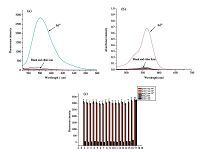
It is an essential feature for a probe to have a high selectivity toward the analyte over the potentially competing species. Shift of fluorescence and absorbance spectra caused competition and selectivity by T1 in the presence of Pd2+ and other competitive metal ions were recorded in Fig. 1a and b,respectively. No obvious fluorescence changes were observed in the presence of other cations (Fig. 1c),such as heavy and transition metal ions (Mn2+,Pb2+,Cd2+,Co2+,Fe3+,Cu2+,Ni2+,Ag+ ,Zn2+,Cr3+,Hg2+),alkali or alkaline-earth metals (Na+,Ca2+,Mg2+,Ba2+) and Al3+.
|
Download:
|
| Fig. 1.Fluorescence (a) and absorbance (b) spectra of T1 (10 μmol/L) in methanol–water (4/6, v/v) solution upon addition of various metal ions (10 μmol/L), λex = 540 nm. (c) Fluorescence intensity changes of T1 (10 μmol/L) upon the addition of various metal ions (10 μmol/L) in the presence of Pd2+ (10 μmol/L) in methanol–water (4/6, v/v) solution. The black bars represent the fluorescence response of T1 and competing ions: (1) Al3+, (2) Na+, (3) Ca2+, (4) Mg2+, (5) Cd2+, (6) Mn2+, (7) Ni2+, (8) Ba2+, (9) Zn2+, (10) Co2+, (11) Cr3+, (12) Pb2+, (13) Ag+, (14) Fe3+, (15) Hg2+, (16) Cu2+, (17) Pd2+. The red bars represent the subsequent addition of 10 mmol/L Pd2+ to the above solutions. | |
However,the F/F0 value increased by more than 100-fold after the addition of Pd2+ at the same concentration. Moreover,Pd2+ caused remarkable enhancement in fluorescence and obvious color changes. The colorimetric and fluorometric behaviors of T1 + Pd2+ complex could be conveniently distinguished from those of the free T1 by a naked eye,as shown in Fig. 2. This strongly suggested that T1 has high selectivity to bivalent palladium ion and can serve as a "naked-eye" probe for Pd2+.

|
Download:
|
| Fig. 2.(a) The color change of the probe T1 in different metal ions ((1) Al3+ [1TD$DIF], (2) Na+, (3) Ca2+, (4) Mg2+, (5) Cd2+, (6) Mn2+, (7) Ni2+, (8) Ba2+, (9) Zn2+, (10) Co2+, (11) Cr3+, (12) Pb2+, (13) Ag+, (14) Fe3+, (15) Hg2+, (16) Cu2+, (17) Pd2+); (b) The fluorescence change of the probe T1 in different metal ions(both 10 μmol/L). | |
A fluorescence and UV titration experiment was carried out to further investigate the interactions between T1 and Pd2+. The stable "spirolactam form" of rhodamine,free T1 represents colorless and no fluorescence response in the range from =0 nm to 750 nm. However,upon the gradual addition of Pd2+ ,a significant enhancement of fluorescence with an emission maximum at 583 nm (Fig. 3a) and an absorbance maximum at =0 nm (Fig. 3b) was observed. It was attributable to the delocalization effects in the xanthene moiety of the rhodamine.

|
Download:
|
| Fig. 3.(a) Fluorescence intensity changes of T1 (10 μmol/L) upon addition of Pd2+ in methanol–water (4/6, v/v) solution. Inset: Changes in the emission intensity at 583 nm. (b) Absorbance intensity changers of T1 (10 μmol/L) upon addition of Pd2+ in methanol–water (4/6, v/v) solution. | |
With the Pd2+ concentration increasing,a continuous increase of fluorescence intensity appeared. After the addition of 1.0 equiv. of Pd2+,the fluorescence intensity showed negligible changes (Fig. 3a,inset). The results clearly indicated that,owing to the addition of Pd(II) to T1,the spirolactam opened and formed a highly delocalized p-conjugated structure. Thus,an obvious and significant enhancement of absorbance and fluorescence was observed.
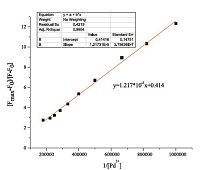
The association constant K was calculated according to the Benesi-Hildebrand equation [49]: (Fmax - F0)/(Fx - F0) = 1 + (1/K) (1/[T]n),where Fmax,F0,and Fx are fluorescence intensities of the probe in the presence of Pd2+ at saturation,free probe,and any intermediate Pd2+ concentration (Fig. 4). The binding constant value was found to be K = 8.77 × 104 (mol/L)-1. The linearity of this plot within the range of 1 μmol/L to 5 μmol/L reveals the formation of a 1:1 clathrate between T1 and Pd2+ (R2 = 0.9954). When the fluorescence enhancement reached the maximum of the probe T1 to Pd2+, F/F0 was as high as over 100-fold,demonstrating that T1 has the excellent capability of detecting Pd2+ both quantitatively and qualitatively.
|
Download:
|
| Fig. 4.Determination of binding constant of T1 (10 μmol/L) with Pd2+ (10 μmol/L) using Benesi–Hildebrand equation. | |
It is well known that the spirolactam ring of the rhodamine derivatives can open in acidic media and then exhibits the strong fluorescence of rhodamine. Therefore,it is necessary to evaluate the effect of pH on the fluorescence of T1. The effects of pH were evaluated in the pH range from 2.0 to 12.0 (Fig. 5). No obvious enhancement of fluorescence at =0 nm was observed within pH 5.0-12.0,suggesting that it was insusceptible to the change of acid-base solution. However,in the presence of Pd2+,a remarkable fluorescence emission band at 583 nm formed under different pH values. It showed the pH values of approximately 4-8 gave the highest responses,suggesting that the probe T1 for Pd2+ could work well in under physiological conditions with a low background response. Therefore,further studies were carried out in a methanol aqueous solution (4/6,v/v) at pH 7.0. Furthermore,the time-dependence of probe T1 fluorescence was also evaluated in the presence of Pd2+ ions. The results showed that,upon the reaction of probe T1 with Pd2+,the fluorescence of all the tested solutions remarkably increased to their maximum value within the first 1 min.

|
Download:
|
| Fig. 5.Effect of pH on probe T1 (10 μmol/L) recognition Pd2+ (pH was adjusted by using methanol–water solutions (v/v = 4/6, the pH of solution was adjusted by aqueous solution of NaOH (1 mol/L) and HCl (1 mol/L). Inset: Effect of time on probe T1 (10 μmol/L) recognition Pd2+ [5TD$DIF] (λex = 540 nm, λem = 583 nm). | |
As is well known,the reversibility is an important property for an excellent probe. Thus,the EDTA—adding experiments were conducted to examine the reversibility of the probe T1. As clearly shown in Fig. 6a,the absorbance decreased when EDTA was added to the mixture containing T1 and Pd2+. Besides,the color of mixture changed from black to colorless and the fluorescence intensity decreased. When Pd2+ was added to the system again,the signals were almost completely reproduced (Fig. 6b) and the colorless solution turned to pink. The above process can be repeated several cycles without significant changes in the fluorescence spectrum.These findings indicated that probe T1 is a reversible fluorescent probe toward Pd2+.

|
Download:
|
| Fig. 6.(a) Absorbance changes of T1 (10 μmol/L) upon the addition of each equiv of EDTA with the presence of Pd2+ in methanol–water (4/6, v/v) solution. (b) Fluorescence intensity of probe T1 + Pd2+as a function of EDTA concentration in methanol–water (4/6, v/v) solution. Inset: Fluorescence intensity at 583 nmfor three cycles of the switching process. | |
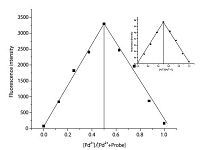
Binding analysis using the method of continuous variations (Job’s plot) was measured. A maximum absorbance at =0 nm and fluorescence emission at 583 nm (Fig. 7) were observed when the molecular fraction of T1 was close to 0.5,which established a 1:1 stoichiometry between T1 and Pd2+. Thus,the most likely binding sites for Pd2+ are the conjugated moieties including phenolic hydroxyl O,imino N,benzoyl hydrazine N and O atoms (Scheme 2). The chelation-induced ring opening of rhodamine spirolactam rather than other possible reactions was likely the mechanism [42, 50].
|
Download:
|
| Fig. 7.Job’s plot of T1 and Pd2+ (The total concentration was 20 μmol/L). | |
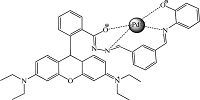
|
Download:
|
| Scheme 2.The most likely binding sites for Pd2+. | |
To further demonstrate the practical applicability of the probe in biological samples,fluorescence imaging experiments were conducted in living cells. The fluorescence images of HepG2 cells were recorded before and after addition of Pd2+,shown in Fig. 8. In the absence of Pd2+,free T1 showed no detectable fluorescence signals in living cells (Fig. 8a). By contrast,after incubation with Pd2+,a bright fluorescence was observed in living cells (Fig. 8c). Bright-field transmission images of cells treated with Pd2+ and probe revealed that the cells were viable throughout the imaging experiments (Fig. 8b). The results suggested that probe T1 can penetrate the cell membrane and can be applied for in vitro imaging of Pd2+ in living cells and potentially in vivo.

|
Download:
|
| Fig. 8.Probe T1 for Pd2+ fluorescence images in HepG2 living cells. Fluorescence images of HepG2 cells treated with probes (20 μmol/L) in either the absence (a) or the presence (c) of 20 μmol/L Pd2+ for 1 h at 37 ℃. (b) Bright-field image of cells shown in panel. (d) Overlay image of (b) and (c). | |
In summary,we have described a new rhodamine-based probe T1,which can give reversible,selective,and sensitive fluorescence enhancement response to Pd2+ via a 1:1 binding mode in methanol aqueous solutions. High selectivity toward Pd2+ is exhibited and little cross-sensitivity is observed to other commonly coexistent metal ions.
The molecular design might greatly contribute to the development of more efficient and useful probes based on the rhodamine scaffold. The value of probes in biological systems is demonstrated by the fluorescence imaging in HepG2 cells. It is anticipated that the probes may be widely used in the studies on the effects of Pd2+ in biological systems.
5. AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No. 21172178).
| [1] | A. Mokhir, A. Kiel, D.P. Herten, R. Kraemer, Fluorescent sensor for Cu2+ with a tunable emission wavelength, Inorg. Chem. 44 (2005) 5661-5666. |
| [2] | X.H. Yang, S. Li, Z.S. Tang, et al., A simple, water-soluble, Fe3+-selective fluorescent probe and its application in bioimaging, Chin. Chem. Lett. 26 (2015) 129-132. |
| [3] | P.J. Jiang, Z.J. Guo, Fluorescent detection of zinc in biological systems: recent development on the design of chemosensors and biosensors, Coord. Chem. Rev. 248 (2004) 205-229. |
| [4] | F. Pina, M.A. Bernardo, E. García-España, Fluorescent chemosensors containing polyamine receptors, Eur. J. Inorg. Chem. 2000 (2000) 2143-2157. |
| [5] | Z.X. Han, B.S. Zhu, T.L.Wu, et al., A fluorescent probe for Hg2+ sensing in solutions and living cells with a wide working pH range, Chin. Chem. Lett. 25 (2014) 73-76. |
| [6] | R. Méallet-Renault, R. Pansu, S. Amigoni-Gerbier, C. Larpent, Metal-chelating nanoparticles as selective fluorescent sensor for Cu2+, Chem. Commun. 20 (2004) 2344-2345. |
| [7] | T. Bura, R. Ziessel, Design, synthesis and redox properties of a fluorene platform linking two different Bodipy dyes, Tetrahedron Lett. 51 (2010) 2875-2879. |
| [8] | L. Pu, Fluorescence of organic molecules in chiral recognition, Chem. Rev. 104 (2004) 1687-1716. |
| [9] | G.W. Gokel, W.M. Leevy, M.E. Weber, Crown ethers: sensors for ions and molecular scaffolds for materials and biological models, Chem. Rev. 104 (2004) 2723-2750. |
| [10] | W.Y. Wong, P.D. Harvey, Recent progress on the photonic properties of conjugated organometallic polymers built upon the trans-bis (para-ethynylbenzene) bis (phosphine) platinum(Ⅱ) chromophore and related derivatives, Macromol. Rapid Commun. 31 (2010) 671-713. |
| [11] | L. Prodi, F. Bolletta, M. Montalti, N. Zaccheroni, Luminescent chemosensors for transition metal ions, Coord. Chem. Rev. 205 (2000) 59-83. |
| [12] | V. Amendola, L. Fabbrizzi, F. Foti, et al., Light-emitting molecular devices based on transition metals, Coord. Chem. Rev. 250 (2006) 273-299. |
| [13] | J. Le Bars, U. Specht, J.S. Bradley, D.G. Blackmond, A catalytic probe of the surface of colloidal palladium particles using heck coupling reactions, Langmuir 15 (1999) 7621-7625. |
| [14] | T. Iwasawa, M. Tokunaga, Y. Obora, Y. Tsuji, Homogeneous palladium catalyst suppressing Pd black formation in air oxidation of alcohols, J. Am. Chem. Soc. 126 (2004) 6554-6555. |
| [15] | M. Lafrance, K. Fagnou, Palladium-catalyzed benzene arylation: incorporation of catalytic pivalic acid as a proton shuttle and a key element in catalyst design, J. Am. Chem. Soc. 128 (2006) 16496-16497. |
| [16] | G. Zeni, R.C. Larock, Synthesis of heterocycles via palladiump π-olefin and π-alkyne chemistry, Chem. Rev. 104 (2004) 2285-2310. |
| [17] | C. Liu, S.K. Zhang, Y.X. Zhang, Z.L. Jin, Arylation of pyridine N-oxides via a ligandfree Suzuki reaction in water, Chin. Chem. Lett. 26 (2015) 55-57. |
| [18] | L.F. Tietze, H. Ila, H.P. Bell, Enantioselective palladium-catalyzed transformations, Chem. Rev. 104 (2004) 3453-3516. |
| [19] | K.C. Nicolaou, P.G. Bulger, D. Sarlah, Palladiumkatalysierte kreuzkupplungen in der totalsynthese, Angew. Chem. Int. Ed. 117 (2005) 4516-4563. |
| [20] | E. Rajanarendar, G. Mohan, E.K. Rao, M. Srinivas, Palladium-catalyzed Suzuki-Miyaura cross-coupling reaction of organoboronic acids with N-protected 4-iodophenyl alanine linked isoxazoles, Chin. Chem. Lett. 20 (2009) 1-4. |
| [21] | X. Chen, K.M. Engle, D.H. Wang, J.Q. Yu, Palladium(Ⅱ)-katalysierte C-H-Aktivierung/C-C-Kreuzkupplung: Vielseitigkeit und Anwendbarkeit, Angew. Chem. Int. Ed. 121 (2009) 5196-5217. |
| [22] | M. Amini, M. Bagherzadeh, S. Rostamnia, Efficient imidazolium salts for palladium-catalyzed Mizoroki-Heck and Suzuki-Miyaura cross-coupling reactions, Chin. Chem. Lett. 24 (2013) 433-436. |
| [23] | V.F. Hodge, M.O. Stallard, Platinum and palladium in roadside dust, Environ. Sci. Technol. 20 (1986) 1058-1060. |
| [24] | T.Z. Liu, S.D. Lee, R.S. Bhatnagar, Toxicity of palladium, Toxicol. Lett. 4 (1979) 469-473. |
| [25] | J.C. Wataha, C.T. Hanks, Biological effects of palladium and risk of using palladium in dental casting alloys, J. Oral Rehabil. 23 (1996) 309-320. |
| [26] | International Programme on Chemical Safety, Palladium: Environmental Health Criteria Series 226, World Health Organization, Geneva, 2002. |
| [27] | K. Van Meel, A. Smekens, M. Behets, P. Kazandjian, R. Van Grieken, Determination of platinum, palladium, and rhodium in automotive catalysts using high-energy secondary target X-ray fluorescence spectrometry, Anal. Chem. 79 (2007) 6383-6389. |
| [28] | M.A. Taher, Z. Daliri, H. Fazelirad, Simultaneous extraction and preconcentration of copper, silver and palladium with modified alumina and their determination by electrothermal atomic absorption spectrometry, Chin. Chem. Lett. 25 (2014) 649-654. |
| [29] | A. Kumar, G.K. Rao, A.K. Singh, Organochalcogen ligands and their palladium(Ⅱ) complexes: synthesis to catalytic activity for Heck coupling, RSC Adv. 2 (2012) 12552-12574. |
| [30] | D. Kalný, A.-M. Albrecht-Gary, J. Havel, Highly sensitive method for palladium(Ⅱ) determination as a porphyrinato complex by capillary zone electrophoresis, Anal. Chim. Acta 439 (2001) 101-105. |
| [31] | R.J.T. Houk, K.J. Wallace, H.S. Hewage, E.V. Anslyn, A colorimetric chemodosimeter for Pd(Ⅱ): a method for detecting residual palladium in cross-coupling reactions, Tetrahedron Lett. 64 (2008) 8271-8278. |
| [32] | E. Unterreitmaier, M. Schuster, Fluorometric detection of heavy metals with Nmethyl-N'-9-(methylanthracene)-N'-benzoylthiourea, Anal. Chim. Acta 309 (1995) 339-344. |
| [33] | K. Kubo, Y. Miyazaki, K. Akutsu, T. Sakurai, Synthesis and emission behavior of double-armed tetrathiacrown carrying two naphthalenes, Heterocycles 51 (1999) 965-968. |
| [34] | B.K. Pal, M.S. Rahman, A nonextractive quenchofluorimetric method for the determination of palladium(Ⅱ) at mg/L levels using bathophenanthroline, Mikrochim. Acta 131 (1999) 139-144. |
| [35] | Y.J. Fang, H. Chen, Z.X. Gao, X.Y. Jin, Studies on the determination of palladium(Ⅱ) by flourescence quenching method with meso-tetra [4-(carboxymethylenoxy) phenyl] porphyrin, Indian J. Chem. Technol. 41A (2002) 521-524. |
| [36] | A. Tamayo, L. Escriche, J. Casabó, B. Covelo, C. Lodeiro, Synthesis, complexation and spectrofluorometric studies of a new NS3 anthracene-containing macrocyclic ligand, Eur. J. Inorg. Chem. 2006 (2006) 2997-3004. |
| [37] | J.R. Matthews, F. Goldoni, H. Kooijman, et al., Metal coordination and aggregation properties of chiral polythiophenes and polythienylethynylenes, Macromol. Rapid Commun. 28 (2007) 1809-1815. |
| [38] | L.P. Duan, Y.F. Xu, X.H. Qian, Highly sensitive and selective Pd2+ sensor of naphthalimide derivative based on complexation with alkynes and thio-heterocyclew, Chem. Commun. 47 (2008) 6339-6341. |
| [39] | T. Schwarze, H. Müller, C. Dosche, et al., Luminescence detection of open-shell transition-metal ions by photoinduced electron transfer controlled by internal charge transfer of a receptor, Angew. Chem. Int. Ed. 46 (2007) 1671-1674. |
| [40] | R.P. Haugland, Handbook of Fluorescent Probes and Research Chemicals, sixth ed., Molecular Probes Inc, Eugene, OR, 1996. |
| [41] | H.N. Kim, M.H. Lee, H.J. Kim, J.S. Kim, J. Yoon, A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions, J. Chem. Soc. Rev. 37 (2008) 1465-1472. |
| [42] | M. Beija, C.A.M. Afonso, J.M.G. Martinho, Synthesis and applications of rhodamine derivatives as fluorescent probes, Chem. Soc. Rev. 38 (2009) 2410-2433. |
| [43] | X.Q. Chen, T. Pradhan, F. Wang, J.S. Kim, J. Yoon, Fluorescent chemosensors based on spiroring-opening of Xanthenes and related derivatives, Chem. Rev. 112 (2012) 1910-1956. |
| [44] | Y.M. Yang, Q. Zhao, W. Feng, F.Y. Li, Luminescent chemodosimeters for bioimaging, Chem. Rev. 113 (2013) 192-270. |
| [45] | B. Valeur, I. Leray, Design principles of fluorescent molecular sensors for cation recognition, Coord. Chem. Rev. 205 (2000) 3-40. |
| [46] | L. Fabbrizzi, M. Licchelli, G. Rabaioli, A. Taglietti, The design of luminescent sensors for anions and ionisable analytes, Coord. Chem. Rev. 205 (2000) 85-108. |
| [47] | C.W. Rogers, M.O. Wolf, Luminescent molecular sensors based on analyte coordination to transition-metal complexes, Coord. Chem. Rev. 233-234 (2002) 341-350. |
| [48] | G.C.R. Ellis-Davies, Neurobiology with caged calcium, Chem. Rev. 108 (2008) 1603-1613. |
| [49] | L.J. Tang, F.F. Li, M.H. Liu, R. Nandhakumar, Single sensor for two metal ions: colorimetric recognition of Cu2+ and fluorescent recognition of Hg2+, Spectrochim. Acta, A: Mol. Biomol. Spectrosc. 78 (2011) 1168-1172. |
| [50] | E.M. Nolan, S.J. Lippard, Tools and tactics for the optical detection of mercuric ion, Chem. Rev. 108 (2008) 3443-3480. |
 2016, Vol.27
2016, Vol.27 


