b Center of Physical and Chemistry Test, Shenyang University of Chemical Technology, Shenyang 110142, China
Singlet oxygen (1O2) is of great importance to various applications of cycloaddition reactions,photodynamic therapy (PDT) and so forth [1, 2, 3]. Especially,PDT is a noninvasive technique for the treatment of a variety of tumors by the combined use of visible or near-infrared light with a photosensitizing drug [4, 5, 6, 7]. The tumor is selectively irradiated with low-energy light of wavelength [8, 9, 10, 11],resulting in excitation of the photosensitizer (PS). Since the singlet oxygen is the key cytotoxic agent in the PDT therapeutic process,the singlet oxygen generation from a photosensitizer is regulated by the efficiency of a spin-forbidden electronic transition from a singlet to a triplet state upon irradiation [12]. Comparing to several types of directly linked BODIPY dimmers without the use of heavy atoms as a PS [13, 14],the heavy atom effect was still advocated and has been a popular and applicable chemical approach to improve intersystem crossing (ISC) to generate the singlet oxygen in several molecules by attaching heavy atoms [15, 16, 17].
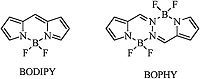
Due to the excellent photochemical properties of boron dipyrromethenes (BODIPY),such as high fluorescence quantum yields,high absorption coefficients and so on,such derivatives have been widely investigated (Fig. 1) [18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31]. Recently,BODIPY based photosensitizers were reported by promoting S1!T1 transition with attached heavy atoms [32].
Very recently,another type of unique pyrrole-BF2-based fluorophore bis(difluoroboron)1,2-bis((1H-pyrrol-2-yl)methylene)- hydrazine (BOPHY) was independently reported by Ziegler and Hao et al. (Fig. 1) [33, 34]. The new fluorescent BOPHY dye can be smoothly obtained by the reaction of pyrrole-2-carboxaldehyde with hydrazine,and followed by complexation with Et3NBF3 ·Et2O. The symmetric structure is composed of four rings at the same plane,including two BF2 units in six-membered chelate rings in the center and two pyrrole units on the periphery (Fig. 1) [33, 34]. The fluorescence quantum yield for the unmodified BOPHY is near 100% [33, 34]. Since the new BOPHY dye has a rigid structure,excellent optical properties promote us to use the BOPHY scaffold as a template for further functionalizations. Our recent research interest lies in the novel BODIPY/aza-BODIPY family of fluorescent dyes and their application [35, 36, 37, 38, 39, 40, 41, 42, 43]. Very recently,our group reported the study on a (p-dimethylamino)- styryl-containing BOPHY as a turn-on fluorescent probe for pH [44],and Ziessel et al. subsequently also reported the BOPHY dye with the intramolecular cascade energy transfer [45]. However,no other modifications on BOPHY dyes were reported. Herein,we report our studies on the modifications on BOPHY dyes by attaching heavy atoms as a photosensitizer for singlet oxygen generation.
|
Download:
|
| Fig. 1.The core structure of BODIPY and BOPHY. | |
Singlet oxygen generation was studied with 1,3-diphenylisobenzofuran (DPBF),a well-known singlet oxygen scavenger,whose maximum absorption at 416 nm diminishes upon reacting with singlet oxygen [46]. However,the reported BOPHY dyes are not suitable as a photosensitizer,due to the overlap of the absorption between BOPHY and DPBF or the no site to attach heavy atoms in the BOPHY structure [33, 34]. To avoid the defect,a tetraphenylcontaining BOPHY dye as a PS was herein designed and found to be highly effective to generate the singlet oxygen.
2. Experimental1HNMR spectra were recorded on a Bruker AVANCE III 500 MHz spectrometer. 1H NMR chemical shifts (δ) are given in ppm downfield from Me4Si,determined by chloroform (δ 7.26). 13C NMR spectra were recorded on a Bruker AVANCE III 125 MHz spectrometer. 13C NMR chemical shifts (δ) are reported in ppm with the internal CDCl3 at δ 77.0 as standard. Toluene solvents were distilled over CaH2. Merck silica gel 60 was used for the column chromatography.
Fluorescence spectra were recorded on a FluoroSENS spectrophotometer. UV/vis spectra were recorded on UV-2=0 spectrophotometer at room temperature. The refractive index of the medium was measured by 2W Abbe’s refractometer at 20 ℃. The fluorescence quantum yield (Φf) of the BOPHY system was calculated using the following relationship (Eq. (1) [47]):
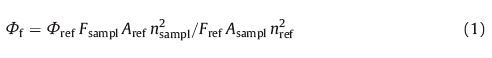
The MO calculations were performed at the DFT level,and the frontier molecular orbitals of BOPHY 1 and 2 at the MP2/6-31G* level with Gaussian 03.
2.1. Synthesis of BOPHY 13,5-Diphenyl-1H-pyrrole-2-carbaldehyde (45 mg,0.18 mmol) and hydrazine hydrate (5.0 mg,0.09 mmol) were dissolved in 20 mL of ethanol. Three drops of acetic acid were added,the solution became yellow. After few seconds,a yellow precipitate formed and the reaction mixture was left to stir at room temperature for an hour. The yellow precipitate was collected by filtration and rinsed with cold ethanol (2 × 10 mL) and dried under vacuum to afford a yellow solid. Then,Et3N (0.5 mL) was added to a solution of this yellow solid in CH2Cl2 (10.0 mL). BF3·Et2O (1.0 mL) was then added dropwise. The reaction mixture was stirred at room temperature overnight. The reaction was quenched with crushed ice,extracted with CH2Cl2,and purified by chromatography on silica gel followed by recrystallization from CH2Cl2/n-hexane to afford BOPHY 1 (25.1 mg,47%) as red solids. Mp: 287.0-288.0 ℃. 1H NMR (500 MHz,CDCl3): δ 8.21 (s,2H),7.84 (d,4H,3J = 7.5 Hz),7.42-7.51 (m,16H),6.82 (s,2H). 13C NMR (125 MHz,CDCl3): δ 152.2,145.2,138.3,132.2,131.4,129.8,129.2,129.0,128.9,128.8,128.4,123.4,117.1. HRMS-MALDI (m/z): [M+Na]+ calcd. for C34H24B2F4N4Na: 609.2021; found 609.2069.
2.2. Synthesis of BOPHY 2BOPHY 1 (21.5 mg,0.036 mmol) was treated with bromine (7.6 mg,0.095 mmol) in dry CCl4 (15 mL) at 30 ℃ under nitrogen for 12 h. The reaction was quenched with water,extracted with CH2Cl2,and purified by chromatography on silica gel followed by recrystallization from CH2Cl2/n-hexane to afford dye 2 (17.2 mg,64%) as yellowish red solids. Mp: 291.0-292.0 ℃. 1H NMR (500 MHz,CDCl3): δ 7.98 (s,2H),7.69 (s,3H),7.47-7.= (m,17H). 13C NMR (125 MHz,CDCl3): δ 149.6,142.9,139.1,130.1,129.9,129.5,129.4,129.1,128.9,128.1,126.7,123.0,106.3. HRMSMALDI (m/z): [M+Na]+ calcd. for C34H22B2Br2F4N4Na: 767.0211; found 767.0356.
2.3. Detection of singlet oxygen by DPBF oxidationSinglet oxygen generation experiment was set up,using a 150W xenon lamp at 0.5 mW/cm2. A toluene solution of photosensitizer (5 × 10-6 mol/L) and 1,3-diphenylisobenzofuran (6 × 10-5 mol/L) was exposed to the monochromatic light by the optical filter at the peak absorption wavelength (500 nm) for 1- 2 min at 25 ℃. The absorbance was measured several times after each irradiation. Reaction of 1,3-diphenylisobenzofuran with singlet oxygen was monitored by the reduction in intensity of the absorption band at 416 nm over 32 min.
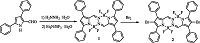
3. Results and discussionSynthesis of the BOPHYs were outlined in Scheme 1. Utilizing 3,5-diphenyl-1H-pyrrole-2-carbaldehyde as the starting material,a centrosymmetric tetraphenyl-containing BOPHY 1 was smoothly obtained in a 47% yield based on the reported literatures [33, 34]. The PS 2 was prepared in a 64% yield by bromination of BOPHY 1 with BΓ2.
|
Download:
|
| Scheme 1.Synthesis of the tetraphenyl-containing BOPHY 1 and the PS 2. | |
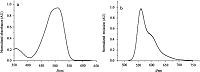
The BOPHY 1 absorbs maximally at 508 nm and emits at 524 nm in CHCl3 (Fig. 2),with the high extinction coefficients (ε = 60,000 L mol-1 cm-1),the narrow full width at half maximum (Fwhm = 78 nm) and the high fluorescence quantum yield (Φf = 0.96),which optical properties are comparable to those of the reported BOPHY dyes [33, 34, 44, 45]. Due to remarkable absorption difference between BOPHY 2 (506 nm) and DPBF (416 nm),the detection of generating singlet-oxygen become possible and was carried out in the following experiments.
|
Download:
|
| Fig. 2.(a) Normalized absorption and (b) fluorescence spectra of 1 in CH2Cl2 at 293 K. | |
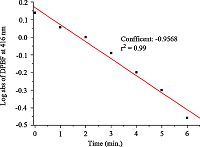
A comparative study of singlet oxygen generation in toluene was performed to assess the ability of the PS 2 to generate singlet oxygen. The solutions were irradiated with monochromatic light [49] at the peak absorption wavelength (500 nm) in the visible region by using a 150W xenon lamp at 0.5 mW/cm2. Singlet oxygen generation was estimated experimentally by DPBF. The decrease of the absorbance band at 416 nm was monitored,caused by the oxidation of DPBF with reactive oxygen species,the singlet oxygen [46]. The experiments were performed at initial concentrations of 5 × 10-6 mol/L of PS 2 and 6 × 10-5 mol/L of DPBF over a period of 32 min. The absorption intensity of DPBF was rapidly decreased 80% at the first 6 min (Fig. 3). A high rate (20.2-fold) of oxygenation of DPBF by 2 was recorded,compared to that of the reference methylene blue (Fig. 4) [26]. Finally,the absorption intensity of DPBF completely disappeared in the following 26 min (Fig. 3). Moreover,in H2O-DMSO (v/v: 9/1) system,the PS 2 also generated the singlet oxygen when the solutions were irradiated with the light (Fig. S1 in Supporting information).

|
Download:
|
| Fig. 3.DPBF (initial concentration at 6 × 10-5 mol/L) degradation profile in toluene by BOPHY 2 (5 × 10-6 mol/L), and the absorption (the bottom curve: λabs = 506 nm) of BOPHY 2 in toluene. Monochromatic light (500 nmat 0.5 mW/cm2) was used. The curves display time-dependent decrease (0, 1, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30 and 32 min) of absorbance at 416 nm by oxidation of DPBF with BOPHY 2. | |
|
Download:
|
| Fig. 4.Comparative DPBF (initial concentration at 6 × 10-5 mol/L) degradation profiles over a period of 6 min in toluene by BOPHY 2. | |
Interestingly,the experimental results showed that PS 2 had high the singlet oxygen production over the 6 min time period (Fig. 3). In addition,no photobleaching of PS 2 was observed during this experiment,based on the absorption intensity (λabs = 506 nm) in toluene (Fig. 3). These are evidences that the PS 2 bearing dibromo substituted groups could be thought to be an excellent PS to be potentially used for the singlet oxygen generation.
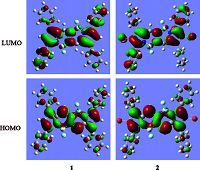
The molecular geometries of BOPHYs 1 and 2 were optimized using density functional theory (DFT) at the B3LYP/6-31G(d) level [50]. The calculated HOMO and LUMO orbital energy levels were summarized in Fig. 5. As shown in Fig. 5,for BOPHY 1 and 2,the HOMO are distributed at the BOPHY core,and the LUMO are localized at the BOPHY core and two phenyl groups of the adjacent Br atoms. In addition,the BOPHY core is almost coplanar. Furthermore,the energy gap between the HOMO and LUMO of the BOPHY 1 (2.95 eV) is slightly smaller than that of PS 2 (2.98 eV) (Fig. 5),which is in good agreement with the red shift in the absorption observed between 1 and 2.
|
Download:
|
| Fig. 5.Frontier molecular orbitals of BOPHYs 1 and 2 at the B3LYP/6-31G(d) level with Gaussian 03. HOMO/LUMO (eV) = -5.58/ - 2.63 for 1; HOMO/LUMO (eV) = -5.80/ - 2.82 for 2. | |
In summary,dibromo substituted BOPHY dye 2 was successfully obtained in 64% yield by the reaction of BOPHY 1 with BΓ2. BOPHY 2 as a photosensitizer was able to high-efficiently generate the singlet oxygen. Singlet oxygen generation by 2 can rapidly accomplish the oxidation of 80% DPBF at the first 6 min. No photobleaching of PS 2 was observed based on the absorption intensity (labs = 506 nm) in toluene. PS 2 having dibromosubstituted group can be thought to be a PS to be potentially used for the singlet oxygen generation. ByMO calculations the HOMO-LUMO band gap for the lowest energy absorption bands of the BOPHY1 issmaller thanthat of PS 2,whichis in good agreement with the red shift in the absorption observed between 1 and 2.
5. AcknowledgmentsThis work was supported by NNSFC (No. 21542004),the Program for Liaoning Excellent Talents in University (No. LJQ2015087),the Public Research Foundation of Liaoning Province for the Cause of Science (No. 2014003009),Educational Department of Liaoning Province (No. L2014170),Science and Technology Key Project of Liaoning Province (No. 2013304007),the Scientific Research Foundation for the Returned Overseas Chinese Scholars,State Education Ministry,and the start-up funds from Shenyang University of Chemical Technology.
6. Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.11. 010.
| [1] | A.G. Leach, K.N. Houk, Diels-Alder and ene reactions of singlet oxygen, nitroso compounds and triazolinediones: transition states and mechanisms from contemporary theory, Chem. Commun. (2002) 1243-1255. |
| [2] | S.B. Brown, E.A. Brown, I. Walker, The present and future role of photodynamic therapy in cancer treatment, Lancet Oncol. 5 (2004) 497-508. |
| [3] | M. Stratakis, M. Orfanopoulos, Regioselectivity in the ene reaction of singlet oxygen with alkenes, Tetrahedron 56 (2000) 1595-1615. |
| [4] | D.E. Dolmans, D. Fukumura, R.K. Jain, Photodynamic therapy for cancer, Nat. Rev. Cancer 3 (2003) 380-387. |
| [5] | I.J. MacDonald, T.J. Dougherty, Basic principles of photodynamic therapy, J. Porphyr. Phthalocyanine 5 (2001) 105-129. |
| [6] | W.M. Sharman, C.M. Allen, J.E. van Lier, Photodynamic therapeutics: basic principles and clinical applications, Drug Discov. Today 4 (1999) 507-517. |
| [7] | R. Bonnett, Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy, Chem. Soc. Rev. 24 (1995) 19-32. |
| [8] | J.V. Frangioni, In vivo near-infrared fluorescence imaging, Curr. Opin. Chem. Biol. 7 (2003) 626-634. |
| [9] | E.M. Sevick-Muraca, J.P. Houston, M. Gurfinkel, Fluorescence-enhanced, near infrared diagnostic imaging with contrast agents, Curr. Opin. Chem. Biol. 6 (2002) 642-650. |
| [10] | C. Sun, J. Yang, L. Li, et al., Advances in the study of luminescence probes for proteins, J. Chromatogr. B 803 (2004) 173-190. |
| [11] | M. Funovics, R. Weissleder, C.H. Tung, Protease sensors for bioimaging, Anal. Bioanal. Chem. 377 (2003) 956-963. |
| [12] | J. Michl, Spin-Orbit Coupling in Biradicals. 1. The 2-electrons-in-2-orbitals model revisited, J. Am. Chem. Soc. 118 (1996) 3568-3579. |
| [13] | Y. Cakmak, S. Kolemen, S. Duman, et al., Designing excited states: theory-guided access to efficient photosensitizers for photodynamic action, Angew. Chem. Int. Ed. 50 (2011) 11937-11941. |
| [14] | W. Pang, X. Zhang, J. Zhou, et al., Modulating the singlet oxygen generation property of meso-b directly linked BODIPY dimers, Chem. Commun. 48 (2012) 5437-5439. |
| [15] | N. Adarsh, R.R. Avirah, D. Ramaiah, Tuning photosensitized singlet oxygen generation efficiency of novel aza-BODIPY dyes, Org. Lett. 12 (2010) 5720-5723. |
| [16] | T. Yogo, Y. Urano, Y. Ishitsuka, et al., Highly efficient and photostable photosensitizer based on BODIPY chromophore, J. Am. Chem. Soc. 127 (2005) 12162-12163. |
| [17] | G. Ulrich, R. Ziessel, A. Harriman, The chemistry of fluorescent bodipy dyes: versatility unsurpassed, Angew. Chem. Int. Ed. 47 (2008) 1184-1201. |
| [18] | A. Loudet, K. Burgess, BODIPY dyes and their derivatives: syntheses and spectroscopic properties, Chem. Rev. 107 (2007) 4891-4932. |
| [19] | T. Kowada, H. Maeda, K. Kikuchi, BODIPY-based probes for the fluorescence imaging of biomolecules in living cells, Chem. Soc. Rev. 44 (2015) 4953-4972. |
| [20] | A. Bessette, G.S. Hanan, Design, synthesis and photophysical studies of dipyrromethene-based materials: insights into their applications in organic photovoltaic devices, Chem. Soc. Rev. 43 (2014) 3342-3405. |
| [21] | H. Lu, J. Mack, Y. Yang, Z. Shen, Structural modification strategies for the rational design of red/NIR region BODIPYs, Chem. Soc. Rev. 43 (2014) 4778-4823. |
| [22] | M.A.T. Rogers, 156. 2,4-Diarylpyrroles. Part I. Synthesis of 2:4-diarylpyrroles and 2:2':4:4'-tetra-arylazadipyrromethines, J. Chem. Soc. (1943) 590-596. |
| [23] | J. Killoran, L. Allen, J. Gallagher,W. Gallagher, D.F. O'Shea, Synthesis of BF2 chelates of tetraarylazadipyrromethenes and evidence for their photodynamic therapeutic behaviour, Chem. Commun. (2002) 1862-1863. |
| [24] | H. Maas, G. Calzaferri, Trapping energy from and injecting energy into dye-zeolite nanoantennae, Angew. Chem. Int. Ed. 41 (2002) 2284-2288. |
| [25] | A. Burghart, L.H. Thoresen, J. Chen, et al., Energy transfer cassettes based on BODIPY dyes, Chem. Commun. (2000) 2203-2204. |
| [26] | A. Gorman, J. Killoran, C. O'Shea, et al., In vitro demonstration of the heavy-atom effect for photodynamic therapy, J. Am. Chem. Soc. 126 (2004) 10619-10631. |
| [27] | R. Gresser, M. Hummert, H. Hartmann, K. Leo, M. Riede, Synthesis and characterization of near-infrared absorbing benzannulated aza-BODIPY dyes, Chem. Eur. J. 17 (2011) 2939-2947. |
| [28] | Z. Zhang, B. Xu, J. Su, et al., Color-tunable solid-state emission of 2,2'-biindenylbased fluorophores, Angew. Chem. Int. Ed. 50 (2011) 11654-11657. |
| [29] | T. Kakui, S. Sugawara, Y. Hirata, S. Kojima, Y. Yamamoto, Anti-aromatic 16π porphyrin-metal complexes with meso-alkyl substituents, Chem. Eur. J. 17 (2011) 7768-7771. |
| [30] | J. Shao, H. Sun, H. Guo, et al., A highly selective red-emitting FRET fluorescent molecular probe derived from BODIPY for the detection of cysteine and homocysteine: an experimental and theoretical study, Chem. Sci. 3 (2012) 1049-1061. |
| [31] | M. Nakamura, H. Tahara, K. Takahashi, et al., π-Fused bis-BODIPY as a candidate for NIR dyes, Org. Biomol. Chem. 10 (2012) 6840-6849. |
| [32] | S. Kim, T.Y. Ohulchanskyy, A. Baev, P.N. Prasad, Synthesis and nanoparticle encapsulation of 3,5-difuranylvinyl-boradiaza-s-indacenes for near-infrared fluorescence imaging, J. Mater. Chem. 19 (2009) 3181-3188. |
| [33] | I.S. Tamgho, A. Hasheminasab, J.T. Engle, V.N. Nemykin, C.J. Ziegler, A new highly fluorescent and symmetric pyrrole-BF2 chromophore: BOPHY, J. Am. Chem. Soc. 136 (2014) 5623-5626. |
| [34] | C. Yu, L. Jiao, P. Zhang, et al., Straightforward synthesis of oligopyrroles through a regioselective S(N)Ar reaction of pyrroles and halogenated boron dipyrrins, Org. Lett. 16 (2014) 1952-1955. |
| [35] | X.D. Jiang, J. Zhang, T. Furuyama, W. Zhao, Development of mono-and di-AcO substituted BODIPYs on the boron center, Org. Lett. 14 (2012) 248-251. |
| [36] | X.D. Jiang, H. Zhang, Y. Zhang, W. Zhao, Development of non-symmetric thiophene-fused BODIPYs, Tetrahedron 68 (2012) 9795-9801. |
| [37] | X.D. Jiang, R. Gao, Y. Yue, G.T. Sun, W. Zhao, A NIR BODIPY dye bearing 3,4,4atrihydroxanthene moieties, Org. Biomol. Chem. 10 (2012) 6861-6865. |
| [38] | X.D. Jiang, Y. Fu, T. Zhang, W. Zhao, Synthesis and properties of NIR aza-BODIPYs with aryl and alkynyl substituents on the boron center, Tetrahedron Lett. 53 (2012) 5703-5706. |
| [39] | X.D. Jiang, D. Xi, J. Zhao, et al., A styryl-containing aza-BODIPY as a near-infrared dye, RSC Adv. 4 (2014) 60970-60973. |
| [40] | X.D. Jiang, J. Zhao, D. Xi, et al., A new water-soluble phosphorus-dipyrromethene and phosphorus-azadipyrromethene dye: PODIPY/aza-PODIPY, Chem. Eur. J. 21 (2015) 6079-6082. |
| [41] | X.D. Jiang, D. Xi, C.L. Sun, et al., Synthesis of a pyrenyl-fused aza-BODIPY as a nearinfrared dye having the absorption maximum at 746 nm, Tetrahedron Lett. 56 (2015) 4868-4870. |
| [42] | X.D. Jiang, H. Yu, J. Zhao, et al., A colorimetric chemosensor based on new watersoluble PODIPY dye for Hg2+ detection, Chin. Chem. Lett. 26 (2015) 1241-1245. |
| [43] | P. Shi, X.D. Jiang, R. Gao, Y. Dou, W. Zhao, Synthesis and application of Vis/NIR dialkyl aminophenylbuta-1,3-dienyl borondipyrromethene dyes, Chin. Chem. Lett. 26 (2015) 834-838. |
| [44] | X.D. Jiang, Y. Su, S. Yue, et al., Synthesis of mono-(p-dimethylamino)styrylcontaining BOPHY dye for a turn-on pH sensor, RSC Adv. 5 (2015) 16735-16739. |
| [45] | Q. Huaulmé, A. Mirloup, P. Retailleau, R. Ziessel, Synthesis of highly functionalized BOPHY chromophores displaying large stokes shifts, Org. Lett. 17 (2015) 2246-2249. |
| [46] | K. Gollnick, A. Griesbeck, Singlet oxygen photooxygenation of furans: Isolation and reactions of (4 + 2)-cycloaddition products (unsaturated sec-ozonides), Tetrahedron 41 (1985) 2057-2068. |
| [47] | A.T.R.Williams, S.A.Winfield, J.N.Miller,Relativefluorescencequantumyieldsusinga computer-controlled luminescence spectrometer, Analyst 108 (1983) 1067-1071. |
| [48] | R.F. Kubin, A.N. Fletcher, Fluorescence quantum yields of some rhodamine dyes, J. Lumin. 27 (1982) 455-462. |
| [49] | L. Huang, X. Cui, B. Therrien, J. Zhao, Energy-funneling-based broadband visiblelight-absorbing bodipy-C60 triads and tetrads as dual functional heavy-atom-free organic triplet photosensitizers for photocatalytic organic reactions, Chem. Eur. J. 19 (2013) 17472-17482. |
| [50] | M.J. Frisch, G.W. Trucks, H.B. Schlegel, et al., Gaussian 03, Gaussian Inc, Pittsburgh, PA, 2003. |
 2016, Vol.27
2016, Vol.27 


