As one of the essential members of G protein-coupled receptors (GPCRs),α1-adrenoceptors (α1-ARs),distributing in various cells,tissues and organs,can convey multiple pivotal extracellular signals. These receptors are categorized into at least three subtypes (α1A,α1B,and α1D) based on their diversities on the biological structure,pharmacological properties,tissue distributions,and signaling pathways [1, 2, 3].
It has been confirmed that α1-ARs are bound up with hypertension,benign prostatic hyperplasia (BPH),and other diseases [4, 5, 6]. In order to prevent and treat diseases connected with α1-ARs anomalously expressed,numerous α1-ARs antagonists have been discovered,such as quinazoline or phenylpiperazine derivatives [7]. Nevertheless,we still face many challenges,which become the stumbling obstacle to studying the biological and pharmacological characteristics of α1-ARs,due to the lack of the three-dimensional crystal structures and tissue-selective antagonists.
Fortunately,with the speedy growth of fluorescence technology,small-molecule fluorescent probes have many merits such as high sensitivity and selectivity for the detection of proteins,enzymes,etc. [8, 9, 10]. Small-molecule fluorescent probes are normally constitutive of two portions,the pharmacophore moiety which could bind to the targets through the receptor-ligand interaction,and the fluorophore which is used to trace the targets by emitting fluorescence signals.
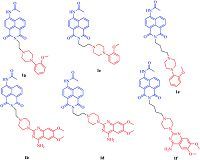
According to our previous work [11, 12, 13, 14, 15],a varied of fluorescent probes for α1-ARs based on naphthalimide were well designed and synthesized (Fig. 1). In this instance,the quinazoline and phenylpiperazine moiety are chosen as the pharmacophore for their high affinity to α1-ARs,and naphthalimide is selected as the fluorophore. With the help of the biological evaluation,we find that our probes showed off the high affinities to α1-ARs and acceptable cell fluorescence imaging potential. It can be expected that these probes could be utilized as useful tools for nowadays high throughput screening of fluorescent competitive substrates in α1-ARs.
|
Download:
|
| Fig. 1.Designed fluorescent probes based on quinazoline and phenylpiperazine for α1-ARs. | |
All materials were purchased from commercial companies (Aladdin and J&K Scientific) and used without further purification. Twice-distilled water was used throughout all experiments. Mass spectra were performed by the analytical and the mass spectrometry facilities in Drug Analysis Center at Shandong University on Agilent Technologies 1100 infinity HPLC,Applied Biosystems API4000. 1H NMR and 13C NMR were recorded on a Bruker 300 MHz NMR spectrometer.
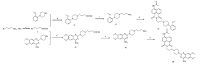
2.2. Synthesis of the probesThe two synthetic routes of these probes were shownin Schemes 1 and 2. The synthesis of key intermediates c2 and d2 began with the CBZ protection of 3-bromopropan-1-amine,and then the nucleophilic substitution and deprotection reactions were conducted (Scheme 1). The other key intermediates a2,e2,b2 and f2 were synthesized through the Gabriel reactions (Scheme 2). All final compounds (1a to 1f) were obtained from the acylation reactions of the key intermediates with a 1,8-naphthalic anhydride.
|
Download:
|
| Scheme 1.Reagents and conditions: (a) Cbz-Cl, 3 mol/L NaOH, CHCl3, overnight; (b) K2CO3, CH3CN, 80 ℃, 5 h, 92%; (c) H2, Pd/C, 30 ℃, overnight, 95%; (d) 4-acetamino-1,8- naphthalic anhydride, CH3CH2OH, 85 ℃, 3 h, 39%–87%. | |

|
Download:
|
| Scheme 2.Reagents and conditions: (a) K2CO3, DMF, 30 ℃, overnight; (b) triethylamine, CH3CN, 85 ℃, 6 h, 60%–88%; (c) (i) hydrazine hydrate, EtOH, 85 ℃, 3 h, (ii) HCl/EtOH; (d) 4-acetamino-1,8-naphathalic anhydride, EtOH, 85 ℃, 3 h, 19%–38%. | |
It is important that the rational fluorescent probes should possess the ideal optical property. After the optical properties of those compounds are measured,the consequences validated that most of the probes owned reasonable optical properties (Table 1). The optical properties were performed on a Thermo-Fisher Varioskan microplate reader by dissolving the probes in 50 mmol/L PBS,pH 7.4. As we can see in Table 1,the maximum absorption wavelength,the excitation wavelength and the fluorescence emission wavelength of all target compounds (1a- 1f) are approximately 3= nm,3= nm and 470 nm,respectively.
|
|
Table 1 Optical properties of compounds 1a–1f. |
Another pivotal characteristic for fluorescent probes is the affinity to the targets (α1-ARs) besides the optical properties. For this reason,the radioligand binding assay for evaluating the affinity of these probes to three different adrenergic receptor subtypes (α1A-,α1B- and α1D-AR) was carried out,in which phentolamine serves as the positive control and atropine served as the negative control. According to the data in Table 2,all probes to α1-ARs had a high affinity at the nanomolar level or even lower the affinity of phenylpiperazine derivatives to the α1-ARs is much higher than that of quinazoline derivatives. However,some recent study indicated that phenylpiperazine derivatives may have high affinity to some subtypes of 5-HT besides α1-ARs [16]. When the pharmacophore is either phenylpiperazine,the compound with a horter linker shows the higher affinity for the targets (Ki1a <Ki1c <Ki1e) ,but for quinazoline pharmacophore,compound 1d with the linker of three carbons shows highest affinity,which is approximately 2-fold more potent than 1b,and the affinity of compund 1f with longer linker is lowest. Phenylpiperazine derivatives show higher affinity to α1A-AR,while quinazoline derivatives show higher affinity to both α1A-AR and α1B-AR. Additionally,lengthening the linker may increase the affinity to α1D-AR of phenylpiperazine derivatives.
|
|
Table 2 The affinity of probes to α1-ARs. |
These compounds showed no difference in fluorescence intensity when incubation with α1-AR proteins,which indicated no "off-on" process happened. We incubated HEK293A cells transfected stably with α1A-AR and α1D-AR with the probes to gain the cell fluorescence imaging. Cells were incubated in DMEM medium complemented with 10% (v/v) fetal bovine serum under the condition of 5% CO2 at 37 ℃.
The cell lineages were reared in 35 mm glass bottom culture dishes (Mat Tek) at 37 ℃ for 24 h. Then cells were washed with DMEM medium and cultured in DMEM medium including the probes for 10 min at 37 ℃. Fluorescence imaging was displayed on a Zeiss Axio Observer A1.
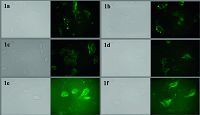
As a result,in Figs. 2 and 3,these cells highly expressing α1A-AR and α1D-AR could be highlighted by fluorescent probes at the nanomolar concentration,which would be an axis for exploiting new longer wavelength probes for α1-adrenergic receptors.

|
Download:
|
| Fig. 2.The fluorescence images of probe 1a–1f to HEK293A-α1A-AR cells (left image is the bright field, right image is the fluorescence image), the concentration of each probe was 50 nmol/L. | |
|
Download:
|
| Fig. 3.The fluorescence images of probe 1a–1f to HEK293A-α1D-AR cells (Left image is the bright field, right image is the fluorescence image), the concentration of each probe was 50 nmol/L. | |
In conclusion,we herein exploited a series of naphthalimidebased small-molecule fluorescent probes with high sensitivity,high affinity and reasonable optical properties for tracking the α1- ARs in living cells. The interesting results laid a solid foundation for further structure-activity relationship optimization and activity screening and these fluorescent probes have been successfully used in visualization of α1-AR in cell imaging. After several cycles of chemical optimization and activity screening,it is possible to pick up an ideal α1-adrenergic receptor fluorescent probes that can be used to study pharmacological and biological characteristics of α1-adrenergic receptor,accelerating the development of α1- adrenergic receptor research. In addition,these probes can be synthesized easily from inexpensive starting materials. It is expected that these probes could be utilized as useful tools for nowadays high throughput screening as fluorescent competitive substrates in α1-ARs.
5. AcknowledgmentsThe present work was supported by grants from the Fok Ying Tong Education Foundation (No. 122036),the Program of New Century Excellent Talents in University (No. NCET-11-0306),the Shandong Natural Science Foundation (No. JQ201019) and the Independent Innovation Foundation of Shandong University,IIFSDU (No. 2010JQ005). Our cell imaging work was performed at the Microscopy Characterization Facility,Shandong University. We also thank Professor You-Yi Zhang from Peking University for her generous gift,the α1A-AR- and α1D-AR-transfected HEK293A cells.
6. Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015. 12.002.
| [1] | C.M. Benning, N. Kyprianou, Quinazoline-derived α1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an α1-adrenoceptor-independent action, Cancer Res. 62 (2002) 597-602. |
| [2] | H.Y. Zhong, K.P. Minneman, α1-Adrenoceptor subtypes, Eur. J. Pharmacol. 375 (1999) 261-276. |
| [3] | W. Li, L. Du, M. Li, Alkaloids and flavonoids as α1-adrenergic receptor antagonists, Curr. Med. Chem. 18 (2011) 4923-4932. |
| [4] | T. Shi, R.J. Gaivin, D.F. McCune, M. Gupta, D.M. Perez, Dominance of the α1B-adrenergic receptor and its subcellular localization in human and TRAMP prostate cancer cell lines, J. Recept. Signal Transduct. Res. 27 (2007) 27-45. |
| [5] | K.S. Jain, J.B. Bariwal, M.K. Kathiravan, et al., Recent advances in selective α1-adrenoreceptor antagonists as antihypertensive agents, Bioorg. Med. Chem. 16 (2008) 4759-4800. |
| [6] | R.R. Ruffolo Jr., J.P. Hieble, Adrenoceptor pharmacology: urogenital applications, Eur. Urol. 36 (1999) 17-22. |
| [7] | N. Kyprianou, C.M. Benning, Suppression of human prostate cancer cell growth by α1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis, Cancer Res. 60 (2000) 4550-4555. |
| [8] | L.Z. Chen, L.P. Du, M.Y. Li, The first inhibitor-based fluorescent imaging probe for aminopeptidase N, Drug Discov. Ther. 7 (2013) 124-125. |
| [9] | Q. Sun, J. Li, W.N. Liu, et al., Non-peptide-based fluorogenic small-molecule probe for elastase, Anal. Chem. 85 (2013) 11304-11311. |
| [10] | X. Wang, L. Cui, N.N. Zhou, et al., A highly selective and sensitive near-infrared fluorescence probe for arylamine N-acetyltransferase 2 in vitro and in vivo, Chem. Sci. 4 (2013) 2936-2940. |
| [11] | M.Y. Li, H. Fang, L. Xia, Pharmacophore-based design, synthesis, biological evaluation, and 3D-QSAR studies of aryl-piperazines as α1-adrenoceptor antagonists, Bioorg. Med. Chem. Lett. 15 (2005) 3216-3219. |
| [12] | L.P. Du, M.Y. Li, Modeling the interactions between α1-adrenergic receptors and their antagonists, Curr. Comput. Aided Drug Des. 6 (2010) 165-178. |
| [13] | M.Y. Li, L. Xia, Rational design, synthesis, biologic evaluation, and structureactivity relationship studies of novel 1-indanone α1-adrenoceptor antagonists, Chem. Biol. Drug Des. 70 (2007) 461-464. |
| [14] | W. Zhang, L.Z. Chen, Z. Ma, L.P. Du, M.Y. Li, Design, synthesis and biological evaluation of naphthalimide based fluorescent probes for α1-adrenergic receptors, Drug Discov. Ther. 8 (2014) 11-17. |
| [15] | W. Zhang, Z. Ma, W.H. Li, et al., Discovery of quinazoline-based fluorescent probes to α1-adrenergic receptors, ACS Med. Chem. Lett. 6 (2015) 502-506. |
| [16] | J. Handzlik, A.J. Bojarski, G. Satała, et al., SAR-studies on the importance of aromatic ring topologies in search for selective 5-HT7 receptor ligands among phenylpiperazine hydantoin derivatives, Eur. J. Med. Chem. 78 (2014) 324-339. |
 2016, Vol.27
2016, Vol.27