b Key Laboratory of Guizhou High Performance Computational Chemistry, Guizhou University, Guiyang 550025, China
Cucurbit[n]urils (Q[n]s or CB[n]s) are a relatively new class of macrocyclic hosts incorporating a rigid hydrophobic cavity and two identical carbonyl fringed portals [1, 2, 3, 4]. The strong chargedipole and hydrogen-bonding interactions,as well as hydrophobic and hydrophilic effects derived from the negative portals and rigid cavities of Q[n]s,endow them with superior molecular recognition and coordination properties in aqueous solution [5, 6, 7, 8, 9, 10, 11],and this has attracted much attention in supramolecular chemistry in the last decades. Recently,our studies [12],along with related results from other researchers [13, 14, 15],have revealed that the electrostatically positive outer surface of Q[n]s could provide a balance of various supramolecular driving forces,such as those involved in C-H. . .p,hydrogen-bonding,and ion-dipole interactions,which could generate numerous novel Q[n]-based supramolecular assemblies and materials. In other words,these results give rise to a broad field of cucurbit[n]urilbased host-guest/coordination chemistry. For example,Kim and co-workers demonstrated that a novel pH-triggered hydrogel exhibiting guest-induced stimuli-responsive behavior could be formed from simple Q[7] molecules [14a]. The driving force for gelation may be the propensity of Q[7] to aggregate through strong hydrogen-bonding interactions between Q[7] portals and hydronium ions,as well as between Q[7] molecules. Later,the same group discovered that hydrogen bonding in outer-surface interactions between Q[6]s could not only function as the sole driving force in the formation of 1D Q[6] porous channels,but also assist the bonding force of the channels in capturing acetylene molecules and carbon dioxide [14b, c],and further giving rise to anisotropic proton conductivity [14d]. Our group has extensively studied the coordination of Q[n]s with metal ions and their supramolecular assemblies in the presence of a third species as a structure inducer [16],in particular the polychloride transitionmetal anions ([MtransClx]n-,Mtrans = Cd,Zn,Cu,Co,Ni,Pt,etc.) [17, 18],which produce the so-called "honeycomb effect" and result in the formation of various coordination polymers [12b]. The driving forcewas suggested to be the dipole interaction of the portal carbonyl carbon atoms with Cl from [MtransClx]n- anions, and hydrogen bonding of the carbonyl oxygen atoms or Cl from [MtransClx]n- anions with methylene or methyne groups on the outer surface of neighboringQ[n]molecules. Typically,six or more [MtransClx]n- anions surround a Q[n] molecule through these interactions and finally two-dimensional honeycomb-like frameworks were formed and thus called "honeycomb effect" [12b],which further attracting metal ions to the portal of the Q[n] molecule,resulting in the formation of coordination complexes of Q[n]s. More recently,we found that larger Q[8]-based porous materials could be obtained solely in the presence of the abovementioned inorganic structure inducers through the "honeycomb effect" [19]. Most importantly,our results further indicated that these polydimensional supramolecular assemblies display potential applications in selective absorption and separation [12, 19, 20].
In the present work,in order to better understand the role and influence of structure inducers such as [CdCl4]2-,noncovalent interactions involved in the self-assembly of Q[n]s have been investigated. We consider here the self-assembly of Q[6] solely in the presence of [CdCl4]2-. X-ray diffraction analysis has revealed that 1D Q[6] porous channels are formed by the noncovalent interactions of C-H. . .Cl (hydrogen bonding of Cl from [CdCl4]2- anions with methine or methylene protons on the back of the Q[6] molecules) and O55C. . .Cl (the dipole interaction between portal carbonyl carbon atoms with Cl from [CdCl4]2- anions),but the "honeycomb effect" induced by [CdCl4]2- anions was not observed in this system. Interestingly,it seems that the "honeycomb effect" and self-assembly of Q[6] can be significantly modified by the presence of the linear guest dibutylamine and [CdCl4]2- anions.
2.ExperimentalThe cucurbit[6]uril was prepared by previously reported procedures [1, 2]. Other chemicals,such as CdCl2×2.5H2O,dibutylamine and HCl were of reagent grade and used without further purification. Elemental analysis was carried out using a EURO EA- 3000 elementanalyzer.
2.1. X-ray crystallographyA suitable single crystal (≥0.2 mm × 0.2 mm × 0.1 mm) was embedded in paraffin oil. The resulting specimen was mounted on a Bruker SMART Apex II CCD diffractometer equipped with a graphite-monochromated Mo Kα radiation source (λ = 0.71073Å , m = 0.828 mm⋅1),which was operated in the v-scan mode at room temperature. Data were corrected for Lorentz and polarization effects by using the SAINT program,and semi-empirical absorption corrections based on equivalent reflections were also applied by using the SADABS program. The structure was elucidated through direct methods and then refined by the full-matrix least-squares method on F2 using SHELXS-97 and SHELXL-97 program packages, respectively [21]. All non-hydrogen atoms were refined anisotropically. Carbon-bound hydrogen atoms were introduced at calculated positions,and were treated as riding atoms with an isotropic displacement parameter equal to 1.2 times that of the parent atom. Most of the water molecules in the compounds were omitted by using the SQUEEZE option of the PLATON program. In addition,the crystallographic data for the reported structures have been deposited at the Cambridge Crystallographic Data Centre as supplementary publication nos. CCDC-1409001 and 1409002.
These data may be obtained free of charge via http://www.ccdc.cam.ac.uk/data_request/cif,by emailing data_request@ccdc.cam. ac.uk,or by contacting the Cambridge Crystallographic Data Centre,12,Union Road,Cambridge CB2 1EZ,UK (fax: +44 1223 336033). 2.2. Preparation of compounds3 Q[6]4(CdCl4)×8(H3O)×46(H2O): CdCl2×2.5H2O (28.0 mg 0.12 mmol) was dissolved in 3.0 mL 3.0 mol/L HCl,and 3.0 mL of 3.0 mol/L HCl containing Q[6] (21.0 mg,0.02 mmol) was then added with stirring. The solution was allowed to stand to allow slow evaporation in air at room temperature. Colorless crystals were obtained from the solution within 3 days (63% yield based on Q[6]×10H2O). Anal. Calcd. for C108H224N72O90Cd4Cl16 (%): C 26.00,H 4.53,N 20.22; found: C 25.93,H 4.57,N 22.14.
Crystal data: C108H224N72O90Cd4Cl16,Mr = 4988.39,triclinic, space group P-1,a = 13.676(3)Å ,b = 16.203(3)Å ,c = 24.364(5)Å , α = 85.908(6)°,β = 88.940(6)°,γ = 67.089(6)°,V = 4959.9(16)Å 3, Z = 1,Dc= 1.670 g/cm3,R1 = 0.0497 (I > 2s(I)),wR2 = 0.1373 (all data),GoF = 0.816. CCDC 1409001.Q[6]×2(C8H20N)×2(CdCl4)×2(H3O)×16(H2O): CdCl2×2.5H2O(28.0mg 0.12 mmol) and C8H19N-HCl (20.0 mg 0.12mmol) were dissolved in 4.0 mL 3.0 mol/L HCl,and 3.0mL of 3.0 mol/L HCl containing Q[6] (21.0 mg,0.02mmol)was then addedwithstirring. The solution was allowed to stand to allow slow evaporation in air at room temperature. Colorless crystals were obtained from the solution within one week (41% yield based on Q[6]×10H2O). Anal. Calcd. for C52H114N26O30Cd2Cl8 (%): C 29.85,H 5.49,N 17.41; found: C 29.76,H 5.54,N 17.32.
Crystal data: C52H114N26O30Cd2Cl8,Mr = 2092.09,triclinic,space group P-1,a = 13.019(5)Å ,b = 14.567(6)Å ,c = 15.137(6)Å ,α = 116.495(11)°,β=92.455(13)(5)°,γ=112.258(13)°,V=2298.8(15)Å 3, Z = 1,Dc= 1.511 g/cm3,R1 = 0.0617 (I > 2s(I)),wR2 = 0.2056 (all data),GoF = 1.037. CCDC 1409002.
3. Results and discussionAs mentioned previously,in aqueous HCl solution,[CdCl4]2- anions have been proved to surround a Q[n] molecule through C-H. . .Cl interactions in the presence a third metal ions and produce a so-called "honeycomb effect" in Q[n]-metal systems [12]. By slow evaporation of the volatiles from a mixture of an aqueous HCl solution (3.0 mol/L) of Q[6] with 6.0 equivalents of CdCl2 over three days at room temperature,colorless single crystals could be obtained. As shown in Fig. 1a,each Q[6] molecule was still surrounded by six [CdCl4]2- anions through two kinds of close noncovalent interactions between the two entities: (1) the dipole interaction between the electron-deficient portal carbonyl carbon sites with Cl from [CdCl4]2- anions (O55C. . .Cl,shown as dark-red dashed lines: distances 3.402-3.332Å ); and (2) hydrogen bonding of Cl from [CdCl4]2- anions with methine or methylene protons on the surface of the Q[6] molecules (C-H. . .Cl,shown as green dashed lines: distances 2.898-2.682Å ). Closer inspection suggested that the noncovalent interactions between Q[6] and [CdCl4]2- anions exhibited two different geometrical features. For example,five Q[6] molecules were tightly assembled by one Cd(1)-derived [CdCl4]2- anion at different angles through four types of noncovalent interactions (Fig. 1b),including seven C-H. . .Cl bonds (a,distances 2.898-2.693Å ); two O55C. . .Cl bonds (b,distances 3.325-3.283Å ); five C-H. . .O bonds (c,distances 2.646-2.484Å ); and one C-H. . .C bond (d,distance 2.646-2.880Å ). On the other hand,as can be seen from Fig. 1c,each of the Cd(2)-derived [CdCl4]2- anions was attached to four Q[6] molecules in similar binding fashions. This result suggested that the tetrahedral geometry of [CdCl4]2- anions in the cucurbituril system plays a very important role in the self-assembly of Q[n]s by providing sufficient noncovalent bonding sites.

|
Download:
|
| Fig. 1.X-ray crystal structure of Q[6] with [CdCl4]2- anions: (a) detailed interactions of a Q[6] molecule with the surrounding [CdCl4]2- anions; (b) detailed interactions of the [Cd(1)Cl4]2- anions with Q[6] molecules; (c) detailed interactions of the [Cd(2)Cl4]2- anions with Q[6] molecules. | |
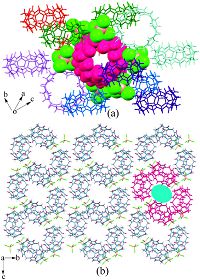
Furthermore,based on the above observations,one Q[6] molecule was ultimately surrounded by twelve Q[6] molecules at different angles through the bridging interactions of the six attached [CdCl4]2- anions (Fig. 2a). Accordingly,the combination of all of these interactions results in the formation of a novel Q[6]- based porous material with one-dimensional channels with an average cross-section of ca. 20Å 2 along the a-axis (Fig. 2b),which were occupied by large amounts of water molecules. Additionally, from the view in Fig. 2b,the "honeycomb effect" that is typically induced and formed by [CdCl4]2- anions in Q[n]-metal systems was not observed in the present study. This phenomenon indicated that the origin of the [CdCl4]2- anion-induced "honeycomb effect" in Q[6]-metal systems may be the result of a subtle synergistic effect of the coordination forces of metals and the noncovalent interactions of [CdCl4]2- anions with the outer surface of Q[6]s.
|
Download:
|
| Fig. 2.X-ray crystal structure of Q[6] with [CdCl4]2- anions: (a) the unit feature of Q[6] molecules with surrounding [CdCl4]2- anions; (b) an overall view of the selfassembly of Q[6] molecules with [CdCl4]2- anions along the a-axis. | |
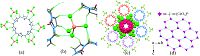
Interestingly,when a linear cationic guest such as dibutylamine hydrochloride (C8H19N-HCl or [NH2(C4H9)2]+Cl⋅) was added to an acidic solution of Q[6]-[CdCl4]2-,the self-assembly of Q[6] was apparently significantly modified and the "honeycomb effect" was observed again. As can be seen from Fig. 3,each Q[6] molecule was surrounded by six [CdCl4]2- anions,but only through the hydrogen bonding of Cl from [CdCl4]2- anions with the methine or methylene protons on the surface of the Q[6] molecules. The overall C-H. . .Cl bond lengths were in the range 2.905-2.666Å . Three Q[6] molecules were simultaneously attached to one [CdCl4]2- anion (Fig. 3b) in a plane through the C-H. . .Cl bonds. As a result,a very regular [CdCl4]2- anion-directed "honeycomb effect" was seen once more and each Q[6] molecule was neatly fixed in the "hollow" by the polychloride transition-metal anions (Fig. 3c and d). In this way,an interesting noncovalent interactionderived macrocyclic host-based single-layer 2D polymer was ultimately formed (Fig. 4).
|
Download:
|
| Fig. 3.X-ray crystal structure of Q[6] with [CdCl4]2- anions in the presence of dibutylamine cations: (a) detailed interactions of a Q[6] molecule with surrounding [CdCl4]2- anions; (b) detailed interactions of the [CdCl4]2-anions with Q[6] molecules; (c) the unit feature of Q[6] molecules with surrounding [CdCl4]2-anions; (d) the [CdCl4]2- honeycomb-like frame. | |

|
Download:
|
| Fig. 4.An overall view of the Q[6]-based single-layer 2D polymer along the a-axis: (a) top view; (b) side view. | |
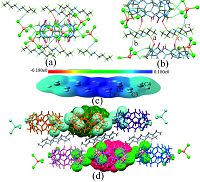
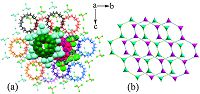
Most importantly,it should be noted that the linear guest dibutylamine hydrochloride seemingly plays an important role in the self-assembly of Q[6] molecules with [CdCl4]2- anions. As shown in Fig. 5,each [CdCl4]2- anion surrounding the Q[6] molecule further interacted with one dibutylamine cation through C-H. . .Cl bonding. For example,six guest molecules were located on the two portals of one Q[6] molecule through C-H. . .O bonds (lengths in the range 2.571-1.949Å ) and C-H. . .Cl bonds (lengths in the range 2.874-2.341Å ),respectively (Fig. 5a). Closer inspection revealed that the guest did not participate in the formation of the single-layer 2D polymer of Q[6] molecules with [CdCl4]2- anions (Fig. 5b),but as the binder induced slight slippage between two adjacent single 2D layers (Fig. 5d). Electrostatic potential surface calculations on the dibutylamine cation guest suggested that the regions around the NH2+ group were significantly positively charged and the alkyl chain was essentially electrostatically neutral (Fig. 5c). In the cucurbituril family,it is well known that the Q[6] molecule shows a preference for encapsulating positively charged ammonium guests in its cavity through ion-dipole interaction. Surprisingly,not only was the dibutylamine cation guest not encapsulated in the cavity of Q[6] in the present system,but also the NH2+ group did not interact with the negatively charged carbonyl groups of Q[6] or the [CdCl4]2- anions. Instead,atoms C1 and C2 of the guest,which bear less positive charge compared to NH2+,were the binding sites to the carbonyl oxygen atoms of Q[6] and Cl from [CdCl4]2-.We propose that these unusual noncovalent interactions may be the major driving forces for the slipped self-assembly of the single layers in the presence of [CdCl4]2- anions as a structure inducer (Fig. 6).
|
Download:
|
| Fig. 5.Detailed interactions between Q[6] molecules, the surrounding [CdCl4]2- anions and the dibutylamine cations (a and b). Electrostatic potentialmap (ESP) for the dibutylaminecationat theB3LYP/6-311G(dandp) levelof theorywithGaussian09(c); Side view of the two layer units in the presence of dibutylamine cations (d). | |
|
Download:
|
| Fig. 6.(a) Overall view along the a-axis of the two Q[6]-based single-layer 2D polymers with slight slippage; (b) the slipped arrangement of the two [CdCl4]2- honeycomb-like frames. | |
As observed in our previous results,the [CdCl4]2- anions induced "honeycomb effect" is not only present in the Q[8]-metal system [12b, 17],but is actually also formed by the Q[8] host. For the latter,we found that Q[8] hosts self-assembled themselves in a zigzag manner [19],which is very similar the assembly fashions of Q[6] or Q[7]-metal coordination based 1D zigzag polymers [16, 21], probably because the larger portal and cavity size allow Q[8] hosts to more easily interact with each other through C-H. . .O55C binding in head to tail fashion under the cooperation of the attached [CdCl4]2- anions. As a result,a stable "honeycomb effect" was formed. For the Q[6]-[CdCl4]2- system,due to the smaller portal and cavity size of Q[6],which makes the host assembly more flexible in the solid state,and the "honeycomb effect" thus is disturbed. However,when the linear guest dibutylamine was added to the Q[6]-[CdCl4]2- solution,as described above,both of Q[6] host and [CdCl4]2- were strongly fixed by the dibutylamine cation guest through C-H. . .O and C-H. . .Cl bonds in the solid structure. Consequently,the self-assembly of Q[6] characteristic of the "honeycomb effect" is again enabled for the system.
4. Conclusion In summary,we have studied here for the first time the selfassembly of Q[6] molecules solely in the presence of [CdCl4]2- anions and in the presence of both a linear cationic organic guest and [CdCl4]2- anions. X-ray diffraction analysis has revealed that 1D Q[6] porous channels were formed by the noncovalent interactions between Q[6] and [CdCl4]2- anions,but the "honeycomb effect" that is typically seen with [CdCl4]2- anions in Q[n]- metal systems was not observed. Most interestingly,it seems that the "honeycomb effect" and the self-assembly of Q[6] with [CdCl4]2- anions can be significantly modified and switched by the presence of the linear cationic guest through some unusual noncovalent interactions. Comparative experiments have revealed that the linear cationic organic guest dibutylamine plays an important role in the self-assembly process of Q[6] molecules with [CdCl4]2- anions. The approach of merging inorganic and organic molecules as hybrid structure inducers in a Q[6] system may well open the way to construct more novel Q[n]-based architectures and materials. We believe that this study will expedite the development of Q[n]-based host-guest/coordination chemistry. Further detailed investigations are ongoing in our laboratories.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21361006),"Chun-Hui" Fund of Chinese Ministry of Education (No. Z2011037) and Guizhou University (No. 20127027).
| [1] | J. Kim, I.S. Jung, S.Y. Kim, et al., New cucurbituril homologues:syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril(n=5, 7, and 8), J. Am. Chem. Soc. 122(2000) 540-541. |
| [2] | A.I. Day, A.P. Arnold, R.J. Blanch, B. Snushall, Controlling factors in the synthesis of cucurbituril and its homologues, J. Org. Chem. 66(2001) 8094-8100. |
| [3] | A.I. Day, R.J. Blanch, A.P. Arnold, et al., A cucurbituril-based gyroscane:a new supramolecular form, Angew. Chem. Int. Ed. 41(2002) 275-277. |
| [4] | X.J. Cheng, L.L. Liang, K. Chen, et al., Twisted cucurbit[14] uril, Angew. Chem. Int. Ed. 5(2013) 7393-7396. |
| [5] | (a) J. Lagona, P. Mukhopadhyay, S. Chakrabarti, L. Isaacs, The cucurbit[n]uril family, Angew. Chem. Int. Ed. 44(2005) 4844-4870;(b) L. Isaacs, Stimuli responsive systems constructed using cucurbit[n]uril-type molecular containers, Acc. Chem. Res. 47(2014) 2052-2062. |
| [6] | K. Kim, N. Selvapalam, Y.H. Ko, et al., Functionalized cucurbiturils and their applications, Chem. Soc. Rev. 36(2007) 267-279. |
| [7] | (a) R.N. Dsouza, U. Pischel, W.M. Nau, Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution, Chem. Rev. 111(2011) 7941-7980;(b) K.I. Assaf, W.M. Nau, Cucurbiturils:from synthesis to high-affinity binding and catalysis, Chem. Soc. Rev. 44(2015) 394-418. |
| [8] | (a) E. Masson, X.X. Ling, R. Joseph, L. Kyeremeh-Mensah, X.Y. Lu, Cucurbituril chemistry:a tale of supramolecular success, RSC Adv. 2(2012) 1213-1247;(b) H. Li, Y.W. Yang, Gold nanoparticles functionalized with supramolecular macrocycles, Chin. Chem. Lett. 24(2013) 545-552. |
| [9] | A.C. Bhasikuttan, H. Pal, J. Mohanty, Cucurbit[n]uril based supramolecular assemblies:tunable physico-chemical properties and their prospects, Chem. Commun. 47(2011) 9959-9971. |
| [10] | (a) Y.L. Liu, H. Yang, Z.Q. Wang, X. Zhang, Cucurbit[8] uril-based supramolecular polymers, Chem. Asian J. 8(2013) 1626-1632;(b) L.H. Wang, Z.J. Zhang, H.Y. Zhang, H.L. Wu, Y. Liu, A twin-axial[5] pseudorotaxane based on cucurbit[8] uril and a-cyclodextrin, Chin. Chem. Lett. 24(2013) 949-952;(c) T.T. Cao, X.Y. Yao, J. Zhang, Q.C. Wang, X. Ma, A cucurbit[8] uril recognized rigid supramolecular polymer with photo-stimulated responsiveness, Chin. Chem. Lett. 26(2015) 867-871. |
| [11] | J. Lü, J.X. Lin, M.N. Cao, R. Cao, Cucurbituril:a promising organic building block for the design of coordination compounds and beyond, Coord. Chem. Rev. 257(2013) 1334-1356. |
| [12] | (a) X.L. Ni, X. Xiao, H. Cong, et al., Self-assemblies based on the "outer-surface interactions" of cucurbit[n]urils:new opportunities for supramolecular architectures and materials, Acc. Chem. Res. 47(2014) 1386-1395;(b) X.L. Ni, X. Xiao, H. Cong, et al., Cucurbit[n]uril-based coordination chemistry:from simple coordination complexes to novel poly-dimensional coordination polymers, Chem. Soc. Rev. 42(2013) 9480-9508. |
| [13] | F. Zhang, T. Yajima, Y.Z. Li, et al., Iodine-assisted assembly of helical coordination polymers of cucurbituril and asymmetric copper(ii) complexes, Angew. Chem. Int. Ed. 44(2005) 3468-3473. |
| [14] | (a) I. Hwang, W.S. Jeon, H.J. Kim, et al., Cucurbit[7] uril:a simple macrocyclic, pHtriggered hydrogelator exhibiting guest-induced stimuli-responsive behavior, Angew. Chem. Int. Ed. 46(2007) 214-217;(b) S. Lim, H. Kim, N. Selvapalam, et al., Cucurbit[6] uril:organic molecular porous material with permanent porosity, exceptional stability, and acetylene sorption properties, Angew. Chem. Int. Ed. 47(2008) 3400-3403;(c) H. Kim, Y. Kim, M. Yoon, et al., Highly selective carbon dioxide sorption in an organic molecular porous material, J. Am. Chem. Soc. 132(2010) 12200-12202;(d) M. Yoon, K. Suh, H. Kim, et al., High and highly anisotropic proton conductivity in organic molecular porous materials, Angew. Chem. Int. Ed. 50(2011) 8016-8019. |
| [15] | P. Thuéry, Lanthanide complexes with cucurbit[n]urils(n=5, 6, 7) and perrhenate ligands:new examples of encapsulation of perrhenate anions, Inorg. Chem. 48(2009) 4497-4513. |
| [16] | L.L. Liang, X.L. Ni, Y. Zhao, et al., Construction of cucurbit[7] uril based tubular nanochannels incorporating associated[CdCl4]2- and lanthanide ions, Inorg. Chem. 52(2013) 1909-1915. |
| [17] | N.N. Ji, X.J. Cheng, L.L. Liang, et al., The synthesis of networks based on the coordination of cucurbit[8] urils and alkali or alkaline earth ions in the presence of the polychloride transition-metal anions, CrystEngComm 15(2013) 7709-7717. |
| [18] | N.N. Ji, X.J. Cheng, Y. Zhao, et al., Hexachloroplatinate(IV) anion induced cucurbituril supramolecular assembly with linear channels, Eur. J. Inorg. Chem. 9(2014) 1435-1438. |
| [19] | N.N. Ji, X.J. Cheng, Y. Zhao, et al., Tetrachloridometallate dianion-induced cucurbit[8] uril supramolecular assemblies with large channels and their potential applications for extraction coating on solid-phase microextraction fibers, Inorg. Chem. 53(2014) 21-23. |
| [20] | X.L. Ni, S.F. Xue, Z. Tao, et al., Advances in the lanthanide metallosupramolecular chemistry of the cucurbit[n]urils, Coord. Chem. Rev. 287(2015) 89-113. |
| [21] | Y. Zhao, L.L. Liang, K. Chen, et al., Inorganic anion-aided coordination oflanthanide metal ions to cucurbituril and supramolecular self-assembly:potential applications in the separation of light lanthanides, CrystEngComm 15(2013) 7987-7998. |
 2016, Vol.27
2016, Vol.27 


