The derivatives of 1H-pyrazole were found to possess activities like antimicrobial,analgesic [1] and antioxidant [2]. On the other hand,fused chromene were proved as a biologically active scaffold and their derivatives carried activities such as antimicrobial [3] and demonstrated excellent antioxidant potency [4]. 2-Pyranone ring system have been also used as antifungals [5, 6],anticonvulsants and antimicrobials [7]. Various catalysts such as, piperidine [8],K2CO3 under microwave irradiation [9],MgO [10], 3-hydroxypropanaminium acetate (HPAA) [11] and many more have been used for the synthesis of chromene and pyran derivatives. These catalysts have some drawbacks like,their reusability,cleanliness of reaction and effectiveness. To enhance the scope of the reaction in the peripheral of green chemistry aspects,we have used a reusable green catalyst,polystyrene supported 7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (PS-TBD), which is a bicyclic guanidine strong base and used in various reactions such as,cyanosilylation of aldehydes,ketones,and imines [12],aryl ethers from phenols,alkyl and aryl halides [13] and addition of dialkylphosphites to unsaturated systems [14], synthesis of aryl triflate and arylnonaflate [15]. None of the reports have been found where PS-TBD has been used for the synthesis of fused chromene and pyran derivatives.
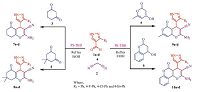
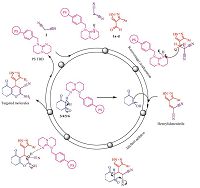
Inspired from the aforementioned reports and our continuous efforts towards the development of novel class of antimicrobial agents [16, 17, 18, 19],We have synthesized a hybrid heterocyclic scaffold carrying 1H-pyrazole and fused chromene or 2-pyranone moiety and synthesized 4H-chromene,pyrano[4,3-b]pyran,pyrano[ 3,2-c]chromene derivatives via one-pot three-component reaction (3CR) between 3-substituted phenyl-1H-pyrazole-4- carbaldehyde 1a-d,malononitrile 2 and corresponding Michael donors (1,3-cyclohexanedione 3/ dimedone 4/ 4-hydroxy-6- methyl-2H-pyran-2-one 5/ and 4-hydroxy-coumarin 6) in presence of 5 mol% PS-TBD (Scheme 1). To explore the medicinal scope of the synthesized molecules,titled derivativeswere screened for their antimicrobial activities by broth microdilution method [20] and antioxidant activity by ferric reducing antioxidant power (FRAP) assay.
|
Download:
|
| Scheme 1.General route for the synthesis of chromene and pyran derivatives. | |
All the reagents and solvents were obtained commercially and used without further purification. All melting points were taken in open capillaries and are uncorrected. For monitoring the progress of all reactions,purity and homogeneity of the synthesized compounds thin-layer chromatography (TLC,on aluminium plates precoated with silica gel,60F254,Merck,Darmstadt,Germany) was used; eluent chloroform: methanol (9:1). UV radiation and/or iodine were used as the visualizing agents. The IR spectra were recorded in KBr on a Perkin-Elmer Spectrum GX FT-IR Spectrophotometer (Perkin-Elmer,USA),only the characteristic peaks are reported in cm-1. Elemental analysis (% C,H,N) was carried out by Perkin-Elmer 2400 series-II elemental analyzer (Perkin-Elmer, USA),all compounds are within ×0.4% of theory specified. 1H NMR and 13C NMR spectra were recorded in DMSO-d6 on a BrukerAvance 400F (MHz) spectrometer (Bruker Scientific Corporation Ltd., Switzerland) using solvent peak as internal standard at 400 MHz and 100 MHz respectively. Chemical shifts are reported in parts per million (ppm). Mass spectra were scanned on a Shimadzu LCMS 2010 spectrometer (Shimadzu,Tokyo,Japan).
2.1. General procedure for the synthesis of targeted compoundsMixture of appropriate 3-substituted phenyl-1H-pyrazole-4- carbaldehyde 1a-d(5 mmol),malononitrile 2 (5 mmol),1,3- cyclohexanedione 3/ dimedone 4/ 4-hydroxy-6-methyl-2H-pyran- 2-one 5/ 4-hydroxy-coumarin 6 (5 mmol) and PS-TBD (5 mol %) in ethanol (5 mL) were charged in 100 mL round bottom flask equipped with condenser. The reaction mixture was stirred at reflux for 1.5 h. After completion of the reaction,as evidenced by TLC,the solid precipitated was dissolved by adding 15 mL chloroform: methanol (1:1). PS-PBD,which remained insoluble was collected by filtration and separately washed with 10 mL acetone and dried in oven to activate for the next batch. The filtrate was concentrated under vacuum to get solid and filtered (yield 86%-95%). The crude product was recrystallized from 50 mL chloroform: methanol (9:1) to get a pure solid sample. PS-TBD maintained its catalytic activity up to five runs.
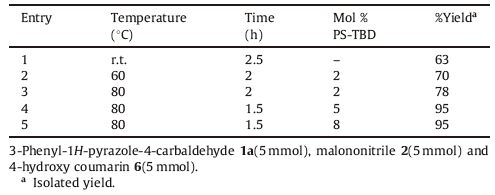
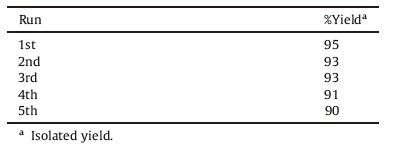
3.Results and discussion 3.1. ChemistryA series of 16 compounds,4H-chromene 7a-d and 8a-d,pyrano[4,3-b]pyran 9a-d and pyrano[3,2-c]chromene 10a-d were synthesized by the plausible reaction mechanism depicted in Scheme 2. The reaction was optimized by varying temperature, time and amount of catalyst (Table 1). We found that reaction of 3- phenyl-1H-pyrazole-4-carbaldehyde 1a,malononitrile 2 and 4- hydroxy-coumarin 6 with 5 mol% PS-TBD,in ethanol at 80 ℃ for 1.5 h gave 95% yield of 10a. Further increasing loading of catalyst didn’t impact on yield. The catalytic activity of PS-TBD was investigated and it was found that PS-TBD maintained its efficiency up to five runs (Table 2).
|
Download:
|
| Scheme 2.Conceivable mechanistic pathway for the title derivatives. | |
|
|
Table 1 Optimization of reaction condition for 10a. |
|
|
Table 2 Activity of reused catalyst for synthesis of 10a. |
The newly synthesized compounds were characterized by 1 H NMR,13C NMR,FT-IR and elemental analysis. The molecular weight of compounds was confirmed by mass spectrometry. Physical,analytical and spectroscopic characterization data of all compounds are given in Supplementary material. 1H NMR (DMSO-d6) spectrum of 10a exhibited a singlet peak around 4.58 ppm stands for H4 proton. Amine and aromatic protons of 10a resonate as multiplets at around 7.21-7.83 ppm. Singlets around 13.93 ppm stands for NH protons and 13CNMR(DMSO-d6) spectrum shows characteristic peak at 27.0 ppm for cyclized carbon,59.2 ppm for C-CN,124.9 ppm for CBBN,153.3 ppm for C- NH2 and 160.1 ppm for C55O,all these peaks support the structure of 10a. The IR spectrum of compound 10a exhibited characteristic absorption bands around 3371-3294 cm-1 and 2206 cm-1 stands for (asym. & sym. stretching) -NH2 and -CN functional groups, respectively. The characteristic absorption band of C55O stretching and C-O-C ether stretching are observed around 1674 cm-1 and 1273 cm-1.
The plausible reaction mechanism can be discussed as,in the first step,heterylidenenitrile forms by the Knoevenagel condensation of 3-substituted phenyl-1H-pyrazole-4-carbaldehyde 1a-d and pre-activated malononitrile 2 in the presence of PS-TBD. PSTBD abstracts the acidic proton of malononitrile and makes it more nuclophilic towards a facial attack on the carbonyl carbon of aldehyde. In second step,Michael addition of activated 3/4/5/6 on heterylidenenitrile results into targeted molecules 7a-d,8a-d,9a-d and 10a-d. The catalyst is used in another cycle for synthesis of targeted molecules.
3.2. Antimicrobial screeningThe antibacterial screening data in Table 3 revealed that compounds 7b,7c,8a,and 10c (MIC = 62.5 μg/mL) were found to have remarkable antibacterial activity when compared with the standard drugs employed,while compound 8b (MIC = 100 μg/mL) was found to have comparable activity with ampicillin (MIC = 100 μg/mL) against gram-negative bacteria E. coli. Against gram-negative bacteria S. typhi,compounds 8b and 10c (MIC = 62.5 μg/mL) were found more potent than ampicillin (MIC = 100 μg/mL),while 7b,7c,and 8d were found equal potency as ampicillin (MIC = 100 μg/mL). Against gram-positive bacteria B. Subtilis,compound 8a (MIC = 62.5 μg/mL) was found to have outstanding activity; compounds 10a and 10b (MIC = 125 μg/mL) showed significant activity; compounds 7c,8d,9a and 10c (MIC = 200 μg/mL) exhibited moderate activity; while compounds 7b and 10d (MIC = 250 μg/mL) were found equally potent when compared with ampicillin (MIC = 250 μg/mL). Against grampositive bacteria S. aureus,compounds 7c,10a and 10b (MIC = 125 μg/mL) were found to have excellent activity; compounds 8c,9c,9d and 10c (MIC = 200 μg/mL) showed better activity; while compounds 7b,7d,8a,9a,9b and 10d (MIC = 250 μg/mL) were found equipotent when compared with ampicillin (MIC = 250 μg/mL).
|
|
Table 3 Antimicrobial screening data of titled compounds. |
Exploration of antifungal screening data Table 3 revealed that, against C. albicans,compounds 10a (MIC = 250 μg/mL) were found to have excellent activity,while compounds 7b,8c,9c and 10c (MIC = 500 μg/mL) were found to have equal potency when compared with griseofulvin (MIC = 500 μg/mL). Against T. rubrum compounds 8c and 9c (MIC = 100 μg/mL) were found to be equally potent to nystatin as well as griseofulvin. Unfortunately,none of the synthesized compounds were found to be active against P. Aeruginosa.
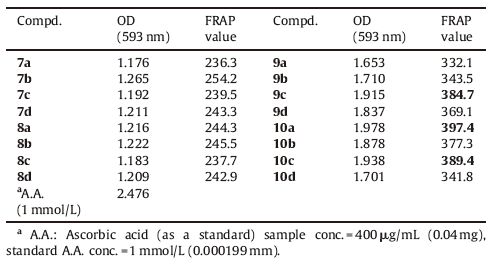
3.3. Antioxidant activityReactive oxygen species are damaging aerobic metabolism. These reactive oxygen species are inactivated by antioxidant agents. An antioxidant agent contains the ability to neutralize free radicals. These free radicals may oxidize nucleic acids,proteins, lipids or DNA and can initiate degenerative disease. In vitro antioxidant activity of newly synthesized compounds was determined chemically using modified ferric reducing antioxidantpower (FRAP)method reported by Benzie and Strain [21]. The absorbance of colour complex Fe (II) TPTZ was measured at 593 nm using ascorbic acid as standard. The results were expressed as ascorbic equivalent (mmol/100 gm compound) shown in Table 4. Compound 9c,10a and 10c possessed excellent FRAP value among this series.

|
|
Table 4 Antioxidant activity data of the series. |
In summary,use of PS-TBD as a reusable catalyst is proved to be very effective for the synthesis of titled compounds. 5 mol% PS-TBD showed good catalytic activity up to five runs with good yield of desired products in less time,that reduce economical cost and environmental pollution. Many of the synthesized compounds exhibited better antimicrobial activity against gram positive bacteria,but showed less potency towards gram negative bacteria. Some of the compounds were found to be equipotent as standard drug in antifungal screening. Compounds 9c,10a and 10c were found to be most efficient antioxidant members in the series.
AcknowledgmentsThe authors are thankful to Head,Department of Chemistry, Sardar Patel University for providing 1 [6TD$DIF]H NMR and 13C NMR spectroscopy,FT-IR and research facilities. We are also thankful to CIST,Sardar Patel University for providing mass spectrometry facilities,SICART,Vallabh Vidyanagar,for elemental analysis and Microcare Laboratory,Surat,for the antimicrobial screening of the compounds reported herein. One of the authors (Nileshkumar D. Vala) is grateful to UGC,New Delhi for BSR Research Fellowship.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.09.020.
| [1] | A.M. Vijesh, A.M. Isloor, P. Shetty, S. Sundershan, H.K. Fun, New pyrazole derivatives containing 1,2,4-triazoles and benzoxazoles as potent antimicrobial and analgesic agents, Eur. J. Med. Chem. 62(2013) 410-415. |
| [2] | A.M. Vijesh, A.M. Isloor, S.K. Peethambar, et al., Hantzschreaction:synthesis and characterization of some new 1,4-dihydropyridine derivatives as potent antimicrobial and antioxidant agents, Eur. J. Med. Chem. 46(2011) 5591-5597. |
| [3] | M.M. Khafagy, A.H.F. AbdEl-Wahab, F.A. Eid, A.M. El-Agrody, Synthesis of halogen derivatives of benzo[h]chromene and benzo[a]anthracene with promising antimicrobial activities, Farmaco 57(2002) 715-722. |
| [4] | K. Mukai, K. Okabe, H. Hosose, Synthesis and stopped-flow investigation of antioxidant activity of tocopherols. finding of new tocopherol derivatives having the highest antioxidant activity among phenolic antioxidants, J. Org. Chem. 54(1989) 557-560. |
| [5] | N. Claydon, M. Allan, J.R. Hanson, A.G. Avent, Antifungal alkyl pyrones of Trichoderma harzianum, Trans. Br. Mycol. Soc. 88(1987) 503-513. |
| [6] | A.Simon,R.W.Dunlop,E.L.Ghisalberti,etal., Trichodermakoningiiproducesapyrone compound with antibiotic properties, Soil. Biol. Biochem. 20(1988) 263-264. |
| [7] | M.D. Aytemir, Ü. Çaliş, M. Ö zalp, Synthesis and evaluation of anticonvulsant and antimicrobial activities of 3-hydroxy-6-methyl-2-substituted 4H-pyran-4-one derivatives, Arch. Pharm. 337(2004) 281-288. |
| [8] | C.B. Sangani, D.C. Mungra, M.P. Patel, R.G. Patel, Synthesis and in vitro antimicrobial screening of new pyrano[4,3-b]pyrane derivatives of 1H-pyrazole, Chin. Chem. Lett. 23(2012) 57-60. |
| [9] | M. Kidwai, S. Saxena, Convenient preparation of pyranobenzopyranes in aqueous media, Synth. Commun. 36(2006) 2737-2742. |
| [10] | M. Seifi, H. Sheibani, High surface area MgO as a highly effective heterogeneous base catalyst for three-component synthesis of tetrahydrobenzopyran and 3,4-dihydropyrano[c]chromene derivatives in aqueous media, Catal. Lett. 126(2008) 275-279. |
| [11] | H.R. Shaterian, A.R. Oveisi, A simple green approach to the synthesis of 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile derivatives catalyzed by 3-hydroxypropanaminium acetate(HPAA) as a new ionic liquid, J. Iran. Chem. Soc. 8(2011) 545-552. |
| [12] | S. Matsukawa, S. Fujikawa, Polystyrene-supported TBD as an efficient and reusable organocatalyst for cyanosilylation of aldehydes, ketones, and imines, Tetrahedron Lett. 53(2012) 1075-1077. |
| [13] | W. Xu, R. Mohan, M.M. Morrissey, Polymer supported bases in combinatorial chemistry:synthesis of aryl ethers from phenols and alkyl halides and aryl halides, Tetrahedron Lett. 38(1997) 7337-7340. |
| [14] | D. Simoni, R. Rondanin, M. Morini, R. Baruchello, F.P. Invidiata, 1,5,7-Triazabicyclo[4. 4.0] dec-1-ene(TBD), 7-methyl-TBD(MTBD) and the Polymer-supported TBD(P-TBD):three efficient catalysts for the nitroaldol(Henry) reaction and for the addition of dialkylphosphites to unsaturated systems, Tetrahedron Lett. 41(2000) 1607-1610. |
| [15] | S. Boisnard, J. Chastanet, J.P. Zhu, A high throughput synthesis of aryl triflate and aryl nonaflate promoted by a polymer supported base(PTBD), Tetrahedron Lett. 40(1999) 7469-7472. |
| [16] | H.H. Jardosh, M.P. Patel, Antimicrobial and antioxidant evaluation of new quinolone based aurone analogs, Arab. J. Chem.(2014), http://dx.doi.org/10.1016/j.arabjc.2014.05.014. |
| [17] | H.H. Jardosh, M.P. Patel, Design and synthesis of biquinolone-isoniazid hybrids as a new class of antitubercular and antimicrobial agents, Eur. J. Med. Chem. 65(2013) 348-359. |
| [18] | H.H. Jardosh, M.P. Patel, Microwave-assisted CAN-catalyzed solvent-free synthesis of N-allylquinolone-based pyrano[4,3-b]chromene and benzopyrano[3,2-c]chromene derivatives and their antimicrobial activity, Med. Chem. Res. 22(2013) 905-915. |
| [19] | H.H. Jardosh, M.P. Patel, Microwave-induced CANpromoted atom-economic synthesis of 1H-benzo[b]xanthene and 4H-benzo[g]chromenederivatives of N-allyl quinolone and their antimicrobial activity, Med. Chem. Res. 22(2013) 2954-2963. |
| [20] | NCCLS(National Committee for Clinical Laboratory Standards), Performance Standards for Antimicrobial Susceptibility Testing:Twelfth Informational Supplement, 2002 ISBN:1-56238-454-6, M100-S12(M7). |
| [21] | I.F. Benzie, J.J. Strain, The ferric reducing ability of plasma(FRAP) as a measure of "Antioxidant Power":the Frap Assay, Anal. Biochem. 239(1996) 70-76. |
 2016, Vol.27
2016, Vol.27