Due to the excellent selectivity,low toxicity and versatile biological activities,the chemistry of nitrogen containing heterocyclic compounds has been a hot topic in pharmaceutical and pesticidal research areas [1]. As one of the five-membered nitrogen-containing heterocycles,1,2,4-triazole introduced into compounds will contribute to a wide-range spectrum of biological activities including anti-inflammatory [2],anticancer [3, 4],antifungal [5],insecticidal [6],and plant growth regulating activities [7]. Furthermore,piperazine ring is one of the important heterocycles and has various properties such as low toxicity,easy forming multiple hydrogen bonds or ionic bonds,and functional effect that modulating drug lipid water partition coefficient and acid-base equilibrium constant. Therefore it is often introduced into some parent structures to enhance the biological properties during the drug design. It has been found that N-substituted piperazine compounds have a wide range of biological activities, such as antimicrobial [8],anticancer [9],herbicidal and insecticidal activities [10],especially they were often used as antimicrobial agent. Mannich bases of 1,2,4-triazole derivatives containing N-substituted piperazine moiety have been reported to possess antifungal activity [11, 12],and some piperazine-containing 4,5-disubstituted-1,2,4-triazole Mannich bases were found to have tuberculostatic activity [13]. However,there are comparatively few literatures about the piperazine-containing compounds for the design and development of agrochemicals.
In our early work,some 1,2,4-triazole Mannich bases containing piperazine and trifluoromethyl groups were synthesized through Mannich reaction,and found to possess significant fungicidal and herbicidal activities [14, 15],which encourage us to carry out further study on this topic. Therefore a series of novel fluorine- and piperazine-containing 1,2,4-triazole thione derivatives were herein designed and synthesized. According to the structure of the lead compound,1-(4-substitutedpyrimidylpiperazin- 1-yl)methyl-4-(substituted)benzylideneamino-3-trifluoromethyl- 1,2,4-triazole-5-thione [14] we reported previously and in view of the importance of fluorine in enhancing bioactivities of various compounds,our intentions were to retain the main structural feature of triazole thione and piperazine moieties of lead compound,change trifluoromethyl group on triazole ring to fluorine-containing aryl group or methyl group,(substituted)benzylideneamino group to phenyl or arylamide group,and study the synthesis and the pesticidal activities of new triazole thione Mannich bases with diverse structures. There are many literatures reported the syntheses of triazole Mannich bases [16, 17, 18],while there is no literature about such kind of compounds bearing an o-fluorophenyl and a phenyl at 3- and 4-position respectively of 1,2,4-triazole ring,and various substituted piperazine moiety (substituted benzyl piperazine,phenyl piperazine,(pyridin-2- yl)piperazine,4,6-disubstituted pyrimidyl piperazine) as amine part of Mannich base,also the symmetric bis(triazole) Mannich bases containing these structural factors. In particular,the Mannich reaction of 1,2,4-triazole thiol containing arylamide group has not been studied so far. In this paper these novel structures designed were successfully synthesized and their herbicidal and fungicidal activities were evaluated.
2. ExperimentalMelting points were determined using an X-4 binocular microscope apparatus and uncorrected. Infrared spectra (IR) (potassium bromide) were recorded on a Bruker Tensor 27 spectrophotometer. 1H NMR and 13C NMR spectra were measured on a Bruker AV-400 instrument (400 MHz) using TMS as an internal standard. Elemental analyses were performed on an elementar Vario EL CUBE elemental analyzer.
Intermediates 4-(4,6-disubstituted pyrimidin-2-yl) piperazine, 4-(substituted)benzylpiperazines,4-phenyl-5-(2-fluorophenyl)- 4H-1,2,4-trizole-3-thiol,4-Amino-5-methyl-4H-1,2,4-trizole-3- thiol were prepared according to the literature [19, 20, 21, 22].
2.1. General synthetic procedures for N-(3-mercapto-5-methyl-4H-1,2,4-triazol-4-yl)-2/3-fluorobenzamide (3)The procedures are similar to that described previously [23]. To the o-fluorobenzoyl chloride (7.9 g,0.5 mol) was added a solution of 4-amino-5-methyl-4H-1,2,4-triazole-3-thiol (6.5 g,0.5 mol) in anhydrous acetonitrile (30 mL) under stirring at 80 ℃. The mixture was refluxed and stirred for 4 h,the solid precipitated was filtered and washed with acetonitrile to give compound 3.
3a: White solid,yield 85%,mp 251-252 ℃; 1H NMR (400 MHz, DMSO-d6): d 13.79 (s,1H,SH),12.02 (s,1H,NH),7.88 (d,1H, J = 7.7 Hz,Ph-H),7.81 (dd,1H,J = 9.5,1.7 Hz,Ph-H),7.67 (m,1H, Ph-H),7.56 (m,1H,Ph-H),2.22 (s,3H,CH3).
3b: White solid,yield 86%,mp 2=-256 ℃; 1H NMR (400 MHz, DMSO-d6):d13.73(s,1H,SH),11.56(s,1H,NH),7.84(m,1H,Ph-H),7.69 (m,1H,Ph-H),7.42 (dd,2H,J = 15.3,8.1 Hz,Ph-H),2.22 (s,3H,CH3).
2.2. General synthetic procedures for 1,2,4-triazole thione derivativesGeneral synthetic procedure for 1-((4-substituted piperazin-1- yl)methyl)-3-(2-fluorophenyl)-4-phenyl-1H-1,2,4-triazole-5(4H)- thione (2a-i): To a solution of 5-(2-fluorophenyl)-4-phenyl-4H- 1,2,4-trizole-3-thiol 1 (0.27 g,1 mmol) in anhydrous ethanol (10 mL),4-substituted piperazine (1 mmol) and 37% formaldehyde (3 mmol) was added,then the resulting solution was stirred at room temperature for 1-2 h. The resulting precipitate was filtered and recrystallized from ethanol and water to give compounds 2a-i.
General synthetic procedure for 1,10-(piperazine-1,4-diylbis( methylene))bis(3-(2-fluorophenyl)-4-phenyl-1H-1,2,4-triazole- 5(4H)-thione) (2j): To a solutionof5-(2-fluorophenyl)-4-phenyl-4H- 1,2,4-trizole-3-thiol 1 (0.4 g,1.48mmol) in anhydrous ethanol (15mL),anhydrous piperazine (0.06 g,0.74mmol) and 37% formaldehyde (4.5mmol)was added,then the reaction solutionwas stirred at room temperature for 20 min. The resulting precipitate was filtered and recrystallized from ethanol to give compound 2j.
General synthetic procedure for N-(1-((4-substituted piperazin- 1-yl)methyl)-3-methyl-5-thioxo-1H-1,2,4-triazol-4(5H)-yl)- 2/3-fluorobenzamide (4a-c,5a-d): The procedure was similar to those of 2a-i. Using N-(3-mercapto-5-methyl-4H-1,2,4-triazol-4- yl)-2/3-fluorobenzamide (3) as trizole material,compounds 4a-c, 5a-d were obtained.
2.3. Herbicidal and fungicidal activity testHerbicidal activity in vivo of compounds were determined by rape root test (Brassica campestris) and barnyardgrass cup test (Echinochloa crusgalli) according to the reported method [24].
Fungicidal activity in vitro of the compounds against Fusarium omysporum,Cercospora arachidicola,Physalospora piricola,Rhizoctonia cerealis,Alternaria solani Sorauer and Gibberella sanbinetti were evaluated via the mycelium growth rate test according to the literature [15].
3. Results and discussion 3.1. Synthesis and spectra characterizationThe synthetic procedures for target compounds 2,4 and 5 were shown in Schemes 1 and 2. The Mannich reaction of 1 or 3 with formaldehyde and substituted piperazine in ethanol at room temperature led to novel Mannich bases with high yields in short time. Using 1,2,4-triazole thiol 1,to react with formaldehyde and various piperazine intermediates which bearing substituted benzyl, or phenyl,or 2-pyridyl,or 4,6-disubstituted pyrimdin-2-yl at 4- position of piperazine,the corresponding 1,2,4-triazole thione derivatives containing various piperazine moieties 2a-i were successfully synthesized. It is worthy to note that Foks et al. prepared the similar compounds from the Mannich reaction of triazole-thiol intermediate with methanol or dioxane as solvent under condition of refluxing (1 h) [13],by contrast,our synthetic procedure during this step for compounds 2a-i was to carry out the reaction in ethanol at room temperature. Using this method,the target compounds can also be obtained in moderate to excellent yields within 1-2 h,which indicates our synthetic reaction has advantage in some extent and this may be a favorable factor during its possible industrial application. In a 2:1 molar ratio of 1,2,4- triazole thiol 1 and piperazine,novel piperazine-containing bis(1,2,4-triazole thione) 2j was prepared conveniently in 89% yield using the same procedure. It was also found that the easy synthetic procedure and satisfactory yield for 2j were similar with those of compounds we reported previously [14],although the substituents at 3- and 4-positions of 1,2,4-triazole were obviously different between two kinds of compounds (3-position: ofluorophenyl vs. trifluoromethyl; 4-position: phenyl vs. (substituted) benzylideneamino). When arylamide-containing 1,2,4-triazole thiol 3 was used,the Mannich reaction also can occur smoothly to give corresponding thione derivatives 4 and 5. Comparing with the usual cases that the (substituted)benzylideneamino or aryl at 4- position of 1,2,4-triazole [16, 17, 25, 26],such novel arylamide case expands the application range for the heterocyclic Mannich reaction. It is known that the intermediates 1 and 3 can exist either as a thiol or the thione tautomeric forms or as an equilibrium mixture of both forms owing to the thioamide structure (-NH- C(=S)). In some cases the mercapto group (-SH) took part in nucleophilic reaction and the sulfoether products obtained [27, 28]. These results in our experiments indicate the thione isomer undertake theMannich reaction via the N-Hat a-position of thiocarbonyl (C=S). Overall these Mannich reactions for such kind of structures (including bis(triazole thione) derivative) exhibit noticeable advantages,such as mild reaction condition,high yield and short reaction time,which may be useful factors for possible application during the development of novel agrochemicals.
The target compounds were identified by melting point,IR,1 H NMR and 13C NMR spectra. The measured elemental analyses were also consistent with the corresponding calculated ones (see Supporting information). In 1H NMR,the resonance signals of CH2 protons neighboring to the triazole ring were observed at δ 5.30-5.40 in Mannich bases 2,while at d 5.10-5.15 in Mannich bases 4 and 5 as a singlet,and the substituted benzyl CH2 proton appeared at δ 3.49-3.61 as a singlet in Mannich bases 2 and 5. The chemical shifts at δ 2.81-3.96 and δ 2.40-3.20 can be ascribed to piperazine ring protons,which were appeared as two broad signals,respectively. Comparing the situation of two active N-H protons in Mannich bases 4 and 5,it was found that the -NH-C=O proton of 4 appeared at d 9.62-10.05,while it appeared at upfield d 8.97-9.16 in 5,which indicates that meta-fluorinated phenyl has better deshielding effect than that of orth-fluorinated. In 13C NMR, almost all carbons of the benzene rings were split by the adjacent fluorine,even the C=O groups in Mannich bases 4 and 5,although very few of them could not be seen clearly. In IR spectra,the characteristic stretching vibrations n(C-F) and n(C=S) appears at 1311-1359 per cm and 11=-1171 per cm,respectively. The spectra of Mannich bases 4 and 5 showed bands at 3213-3287 per cm for N-H stretching and 1678-1689 per cm for C=O stretching.

|
Download:
|
| Scheme 1.General synthetic route for title compounds 2. Reagents and conditions: (a) SOCl2, EtOH, refluxing, 3 h; (b) 85% hydrazine hydrate, EtOH, 6 h; (c) PhNCS, EtOH, refluxing, 3 h; (d) NaOH solution, HCl, refluxing, 4 h; (e) 37% CH2O, 4-substitutied piperazine, EtOH, r.t., 1–2 h; (f) 37% CH2O, anhydrous piperazine, EtOH, r.t., 20 min. | |

|
Download:
|
| Scheme 2.General synthetic route for title compounds |
|
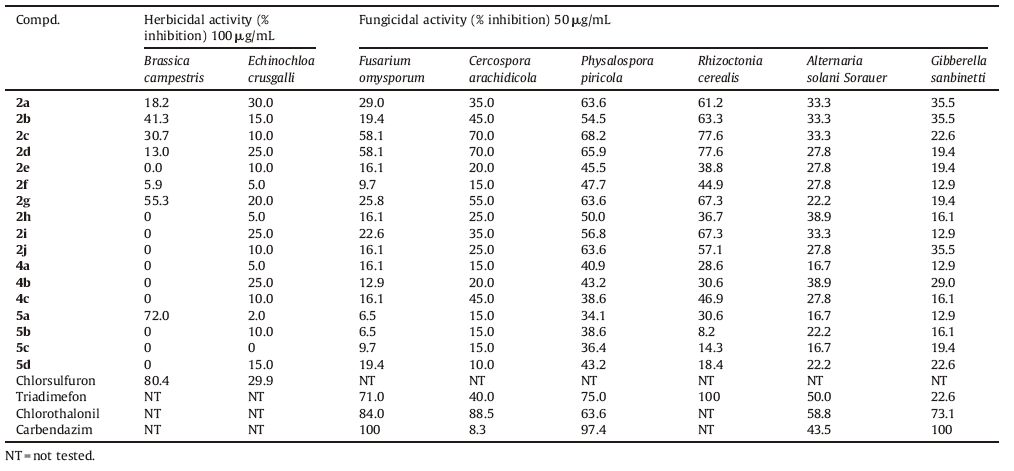
As shown in Table 1,most of the title compounds showed rather weak herbicidal activity based on the rape (B. campestris) root test compared with commercial herbicide chlorsulfuron at a concentration of 100 μg/mL,however compound 5a exhibited 72.0% of inhibitory activity and was comparable with the control. For the barnyardgrass (E. crusgalli) cup tests,compounds did not show potent herbicidal activity at 100 μg/mL.
The in vitro fungicidal results of compounds against six fungi were listed in Table 1. It was found that most compounds showed obvious growth inhibitory activity against all test fungi at 50 μg/mL. For C. arachidicola,several compounds were more effective than the control Triadimefon,e.g. 2c and 2d showed 70.0% of inhibitory activity,which data were higher than that of Triadimefon (40.0%) against C. arachidicola. For P. piricola, compounds 2a,2c,2d,2g and 2j exhibited 63.6%,68.2%,65.9%, 63.6%,and 63.6% activity,respectively,and had similar inhibitory level with that of the control Chlorothalonil (63.6%). For R. cerealis, compounds 2b-2d,2g and 2i possessed 63.3%-77.6% inhibitory activity. Although these compounds were less effective than the control,they showed favorable and significant fungicidal activity against the two fungi,which will provide important structural information for designing novel fungicides. As we see in Table 1, compounds 2 seemed to be more active against test fungi than compounds 4 and 5 broadly. It was worthy to note that 2c and 2d exhibited more favorable fungicidal activity and broad fungicidal spectrum than others,and could be novel lead structures for further optimization during our fungicides innovation program.
|
|
Table 1 The biological activities of title compounds. |
In conclusion,series of fluorine- and piperazine-containing 1,2,4-triazole thione derivatives have been synthesized by the Mannich reaction of triazole intermediates with formaldehyde and various substituted piperazines under mild conditions in short time and high yields. The preliminary bioassays for 17 novel compounds showed that most of the compounds exhibit weak herbicidal activity against B. campestris and E. crusgalli at 100 μg/mL,while several compounds have significant fungicidal activities against C. arachidicola,P. piricola and R. cerealis at 50 μg/mL. Among which, compounds 2 are broadly more active than compounds 4 and 5, especially 2c and 2d showed broad activities and could be used as novel fungicidal lead structures for further optimization.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21372133) and "111" Project of Ministry of Education of China (No. B06005). We thank Dr. Yong-Hong Li of the Biological Assay Center,Nankai University,for herbicidal tests of compounds.
| [1] | J. Zhang, G.M. Xiao, Study progress in nitrogenous heterocyclic compounds, Petrochem. Technol. 40(2011) 579-584. |
| [2] | J.Y. Xu, Y. Zeng, B. Jiang, et al., Synthesis, anti-inflammatory activities and SAR studies of 1,5-diaryl substituted-1,2,4-triazoles, Chin. J. Med. Chem. 18(2008) 321-328. |
| [3] | Q.H. Li, G. Zhang, Y. Ding, et al., Synthesis and anti-tumor activities of novel triazole Schiff-base derivatives, J. Southwest Univ. Natl:Natl. Sci. Ed. 40(2014) 826-832. |
| [4] | Y.G. Zheng, W. Xue, Q.Q. Guo, et al., Synthesis and antitumor activity of 5,6-2H-[1,2,4]-triazolo[3,4-b][1,3,4] thiadiazine derivatives, Chin. J. Org. Chem. 31(2011) 912-916. |
| [5] | Z.X. Feng, W.N. Zhang, Y.J. Zhou, et al., Synthesis and antifungal activities of 1-[2-(N-methyl-N-substituted-benzyl)amino-2-(4-tert-butylphenyl)ethyl]-1H-1,2,4-triazoles, Chem. J. Chin. Univ. 21(2000) 1221-1226. |
| [6] | K. Yin, L.H. Jiang, H.X. Zhou, Y. Huang, J.N. Xiang, Synthesis and insecticidal activity of 2-perfluoroalkyl-substituted or glucopyranosyl-substituted 2,4-dihydro-1,2,4-triazole-3-thione Schiff base, Chin. J. Org. Chem. 28(2008) 1016-1023. |
| [7] | X.Y. Zhao, Y.X. Gong, Z.W. Zhang, et al., Synthesis and plant growth regulating activity of N-5-(3-carboxy-1,2,4-triazolyl)-N'-aroyl urea, Chin. J. Appl. Chem. 20(2003) 594-596. |
| [8] | Q. Wu, Z.C. Wang, X. Wei, W. Xue, Synthesis and antibacterial activities of 1-substituted-4-[5-(4-substitutedphenyl)-1,3,4-thiadiazol-2-sulfonyl]piperazine derivatives, Chin. J. Synth. Chem. 22(2014) 429-434. |
| [9] | D.H. Jiang, M. Huang, Design and synthesis of thieno[3,2-d]pyrimidine derivatives containing a piperazine unit as anticancer agents, Chem. Reag. 34(2012) 797-799. |
| [10] | G.Y. Li, S.G. Yan, S. Jiang, et al., Synthesis of piperazine derivatives containing pyridinemethyl/thiazolemethyl and their biological activities, Chin. J. Org. Chem. 28(2008) 2001-2006. |
| [11] | S. Liang, C.M. Liu, Y.S. Jin, et al., Synthesis and the antifungal activity of 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-[(4-substituted)-piperazine-1-yl]-2-propanols, Chin. J. Med. Chem. 14(2004) 71-75. |
| [12] | G.M. Ye, Q.Y. Sun, Y.Y. Jiang, et al., Synthesis and antifungal activity of 1-(1H-1,2,4-triazole-1-yl)-2-(2,4-difluorophenyl)-3-[(4-substituted-piperazine)-1-yl]-2-propanols, Acad. J. Sec. Mil. Med. Univ. 26(2005) 1161-1164. |
| [13] | H. Foks, M. Janowiec, Z. Zwolska, E. Augustynowicz-Kopeć, Synthesis and tuberculostatic activity of some 2-piperazinmethylene derivatives 1,2,4-triazole-3-thiones, Phosphorus Sulfur Silicon Relat. Elem. 180(2005) 537-543. |
| [14] | B.L. Wang, Y.X. Shi, Y. Ma, et al., Synthesis and biological activity of some novel trifluoromethyl-substituted 1,2,4-triazole and bis(1,2,4-triazole) Mannich bases containing piperazine rings, J. Agric. Food Chem. 58(2010) 5515-5522. |
| [15] | B.L. Wang, X.H. Liu, X.L. Zhang, et al., Synthesis, structure and biological activity of novel 1,2,4-triazole Mannich bases containing a substituted benzylpiperazine moiety, Chem. Biol. Drug Des. 78(2011) 42-49. |
| [16] | A. Demirbas, D. Sahin, N. Demirbas, S.A. Karaoglu, Synthesis of some new 1,3,4-thiadiazol-2-ylmethyl-1,2,4-triazole derivatives and investigation of their antimicrobial activities, Eur. J. Med. Chem. 44(2009) 2896-2903. |
| [17] | B.S. Holla, B.S. Rao, K. Shridhara, P.M. Akberali, Studies on arylfuran derivatives:Part XI. Synthesis, characterisation and biological studies on some Mannich bases carrying 2,4-dichlorophenylfurfural moiety, Il Farmaco 55(2000) 338-344. |
| [18] | K.V. Sujith, J.N. Rao, P. Shetty, B. Kalluraya, Regioselective reaction:synthesis and pharmacological study of Mannich bases containing ibuprofen moiety, Eur. J. Med. Chem. 44(2009) 3697-3702. |
| [19] | P. Zlatoidský, T. Maliar, Synthesis of 1-(4-acyloxybenzoyloxyacetyl)-4-alkylpiperazines and 1-(4-acyloxybenzoyl)-4-alkylpiperazines as inhibitors of chymotrypsin, Eur. J. Med. Chem. 31(1996) 669-674. |
| [20] | R.R. Adams, F.C. Whitmore, Heterocyclic basic compounds. IV. 2-aminoalkylamino-pyrimidines, J. Am. Chem. Soc. 67(1945) 735-738. |
| [21] | J.Y. Tong, Y.X. Shi, X.H. Liu, N.B. Sun, B.J. Li, Synthesis and fungicidal activity of 1,2,4-triazole derivatives containing 2-fluorophenyl moiety, Chin. J. Org. Chem. 32(2012) 2373-2377. |
| [22] | C.Y. Liu, Q.Q. Zhao, J. Li, Synthesis study of new Schiff base containing thiocabamide group derivatives of 3-argl-4-amino-5-mercapto-1,2,4-triazoles, Chem. Reag. 23(2001) 344-345. |
| [23] | S.J. Xue, C.S. Xiang, S.R. Yu, et al., Synthesis and crystal structure of 5-(p-tolyl)-4-[2-(2,4-dichlorophenoxy)acetamido]-1,2,4-triazole-3-thione ethyl acetate solvate, Chin. J. Struct. Chem. 27(2008) 389-393. |
| [24] | B.L. Wang, R.G. Duggleby, Z.M. Li, et al., Synthesis, crystal structure and herbicidal activity of mimics of intermediates of the KARI reaction, Pest Manag. Sci. 61(2005) 407-412. |
| [25] | H. Bayrak, A. Demirbas, S.A. Karaoglu, N. Demirbas, Synthesis of some new 1,2,4-triazoles, their Mannich and Schiff bases and evaluation of their antimicrobial activities, Eur. J. Med. Chem. 44(2009) 1057-1066. |
| [26] | S. Ceylan, H. Bektas, H. Bayrak, et al., Syntheses and biological activities of new hybrid molecules containing different heterocyclic moieties, Arch. Pharm. 346(2013) 743-756. |
| [27] | F. Micheli, G. Bonanomi, F.E. Blaney, et al., 1,2,4-Triazol-3-yl-thiopropyl-tetrahydrobenzazepines:a series of potent and selective dopamine D3 receptor antagonists, J. Med. Chem. 50(2007) 5076-5089. |
| [28] | P. Polucci, P. Magnaghi, M. Angiolini, et al., Alkylsulfanyl-1,2,4-triazoles, a new class of allosteric valosine containing protein inhibitors. Synthesis and structureactivity relationships, J. Med. Chem. 56(2013) 437-450. |
 2016, Vol.27
2016, Vol.27