Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions of aryl halides with aryl boronic acids have been accredited as one of the most powerful and convenient synthetic tools to synthesize unsymmetrical bi-aryl compounds [1]. Recently,as a new generation of heterogeneous catalysts for the Suzuki coupling reaction,immobilization of Pd nanoparticles on solid supports has received considerable attention because of their superior catalytic performance,good stability,ease of separation and satisfactory reusability in comparison to the traditional homogeneous catalysts, such as Pd(OAc)2 and PdCl2 [2, 3, 4, 5, 6]. Generally,the solid supports,especially carbon nanomaterials such as activated carbon [7],carbon molecular sieve [8],fullerene [9],carbon nanotube [10],nanofiber and graphene [11],have been used as the support for heterogeneous palladium catalysts [12, 13]. However, noble metals (e.g.,Pt,Pd,and Ru) deposited on these carbon nanomaterials can easily leach during the catalytic processes because the interaction between the metal nanoparticles and the carbon support is weak,and also the chemical or catalytic properties of the traditional carbon supports do not always satisfy the sharply increasing demands of catalysis [9, 14].
Currently,nitrogen-doped porous carbon (N-doped PC),as a kind of novel material,has attracted considerable attention. NDoped PC promises a wide range of applications in many fields because the incorporation of nitrogen atoms in the carbon architecture can enhance chemical,electrical,and functional properties. Furthermore,thanks to the activation of neighboring carbon atoms caused by the electron affinity of nitrogen,the presence of doped nitrogen heteroatoms on carbon support could stabilize the noble metal (e.g.,Pd,Pt) nanoparticles,which is rather beneficial for heterogeneous catalysts [15, 16]. In general,N-doped PC has conventionally been synthesized via chemical vapor deposition,arc-discharge/vaporization approach and plasma treatment under an NH3 atmosphere or in the presence of other nitrogen sources such as pyridine and acetonitrile [17]. However, these methods often encounter one or more shortcomings,such as rigorous,tedious and multistep preparative processes,and the resulting N-doped PC materials often have poor control to the loading amount of nitrogen,and tend to have low chemical homogeneity and disordered structures [18].
Recently,metal-organic frameworks (MOFs) resulting from periodically arranged organometallic complexes have emerged as a new family of solid matrices for use as hard templates for the casting of porous carbons owing to their high specific surface area and porosity,chemical tunability,and well-defined pore structure [14, 19, 20, 21, 22, 23, 24, 25]. Moreover,since the organic ligands contain various types of atoms (N,O,P or S,etc.) other than carbon,MOFs might be a kindof promisingcandidate for the fabrication of heteroatom-doped nanoporous carbon materials with uniformly distributed catalytic centers and a highly active site density. So for,several MOFs containing nitrogen have been demonstrated as promising selfsacrificial templates to afford capacitance [17, 24, 30] and sensing [28, 31]. Zhao et al. synthesized an N-doped PC material by direct carbonization of an amine to highly N-doped PC materials [20, 26, 27]. The N-doped PC materials have found many applications in gas adsorption [28, 29],electrochemical functionalized aluminum- based MOF (amino-MIL-53). The obtained material was used as metal-free,electro-catalysts for oxygen reduction reactions [19]. N-doped PC derived from zeolitic imidazolate framework 8 (ZIF-8) nanocrystals has also been used as efficient electro-catalysts for oxygen reduction reactions [18]. However,the application of MOFderived N-doped PC in heterogeneous catalysis is still very limited.
In continuation of our interest in exploring efficient catalysts for organic transformations [2, 32, 33],in this paper,N-doped PC was fabricated by a one-step,direct,carbonization of ZIF-8,which serves as the nitrogen source,the carbon source,as well as the hard template. After that,Pd nanoparticles were supported on the asprepared N-doped PC. In order to evaluate the catalytic activity of the catalyst,the Suzuki-Miyaura coupling reaction was chosen as the model reaction.
2. Experimental2-Methylimidazole (99%,MIm),aryl boronic acids,aryl halides, and palladium chloride (PdCl2) were purchased from Aladdin Reagent Limited Company and used as received. Sodium hydroxide, Zn(NO3)2⋅6H2O (99%) and sodium borohydride were obtained from Chengxin Chemical Reagents Company (Baoding,China) and used without further purification. Methanol and ethanol were provided by the Boaixin Co.,Ltd. (Baoding,China).
The size and morphology of the catalyst was observed by scanning electron microscopy (SEM) using a FEI Quanta 200F field emission electron microscope operated at 30 kV. The transmission electron microscopy (TEM) was acquired using a JEOL model JEM- 2011(HR) at 200 kV. The X-ray diffraction (XRD) patterns of the samples were recorded with a Rigaku D/max 2500 X-ray diffractometer using Cu Kα radiation (40 kV,150 mA) in the range 2θ = 10-80°. The Brunauer-Emmett-Teller (BET) surface area and porous structure were measured using V-Sorb 2800P. After the samples were degassed in vacuum at 120 ℃ for 6 h,the nitrogen adsorption and desorption isotherms were measured at 77 K. X-ray photoelectron spectroscopy (XPS) was performed with a PHI 1600 spectroscope using Mg Kα X-ray source for excitation. The Pd content of the catalyst was determined by means of inductively coupled plasma atomic emission spectroscopy (ICP-AES) on Thermo Elemental IRIS Intrepid II.
The ZIF-8 nanocrystals were prepared according to the reported synthesis protocol [18]. Typically,a solution of Zn(NO3)2⋅6H2O (2.348 g,7.89 mmol) in 160 mL of methanol is rapidly poured into a solution of 2-methylimidazole (5.192 g,63.24 mmol) in 160 mL of methanol under magnetic stirring. The mixture was stirred at room temperature for 1 h. The solid product was separated from the milky colloidal dispersion by centrifugation. After washing with methanol three times,the white ZIF-8 product was dried in a vacuum at 60 ℃ for 2 h. For the synthesis of N-doped PC,the carbonization of the ZIF-8 was performed at 700,800,900,1000 ℃ for 8 h with an Ar flow,respectively. The resulting products were denoted as N-doped PC-700,N-doped PC-800,N-doped PC-900, and N-doped PC-1000,respectively.
Pd nanoparticles were immobilized on the N-doped PC by an impregnation method. A 100 mg N-doped PC sample was dispersed in 10 mL (1 mg/mL) chlorine palladium acid solution, and then the solution was stirred overnight. After filtration,the solid was washed with water and dried under vacuum at 70 ℃. The resultant was dispersed in 10 mL water,then 11 mg sodium borohyride (NaBH4,0.027 mmol) was added to the solution. The pH of the mixture was adjusted to 10 with a 10% sodium hydroxide solution and the reaction was carried out at 98 ℃ for 2 h. The obtained solid,N-doped PC-Pd,was washed with water and dried in vacuum at 50 ℃.
For the preparation of Zn-free N-doped PC-900-Pd,100 mg of N-doped PC-900 powder was suspended in 35 mL of 10% HCl solution and held for 5 h,and then the mixture was filtered and washed with distilled water. Finally,the N-doped PC-900 without ZnO or Zn was obtained by vacuum drying at 80 ℃ overnight.
The Zn-free N-doped PC-900-Pd was fabricated according to the same procedure mentioned above except that 50 mg N-doped PC was replaced by 50 mg Zn-free N-doped-900-PC.
To a 25 mL round-bottom flask,aryl halide (0.5 mmol), phenylboronic acid (0.6 mmol),base (1.5 mmol),solvents (EtOH/ H2O = 1:1,v/v,4 mL) and a certain amount of the catalyst N-doped PC-Pd were added and stirred at r.t. After a desired reaction time, the mixture was diluted with 10 mL of H2O and extracted with diethyl ether (3×10 mL). The organic layers were combined,dried over anhydrous MgSO4 and filtered. Then the filtrate was concentrated by vacuum. The pure products were obtained by flash chromatography using petroleum ether:ethyl acetate (9:1) as the eluent. The preparation process of the catalyst and the Suzuki coupling reaction were illustrated in Scheme 1.
|
Download:
|
| Scheme 1.The preparation process of the catalyst and the Suzuki coupling reaction. | |
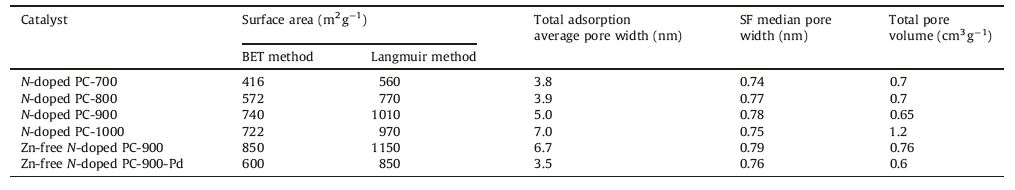
In order to explore the pore structure of N-doped PC samples, the nitrogen adsorption and desorption isotherms were recorded at 77 K. As detailed in Table 1,with increasing the carbonization temperature from 700 ℃ to 900 ℃,the experimental multipoint BET surface area of the N-doped PC increased from 416 to 740 cm3 g-1 and the total adsorption average pore width also increased to 5.0 nm. However,the BET then slightly decreased to 720 cm3 g-1 and the total adsorption average pore width increased to 7.0 nm when the carbonization temperature was 1000 ℃. The reason may probably be due to the cleavage of C-N and escape of nitrogen as well as the further graphitization of the carbon framework at high temperature [18, 34]. Additionally,it is also worth noting that the BET surface area of N-doped PC-900 increased from 740 to 850 cm3 g-1 after the removal of Zn impurities by washing with HCl. Since the N-doped PC was fabricated from the ZIF-8 precursor originating from a carbonization process,the morphology and pore texture of the parent MOF may well be inherited and even enhanced [18].
|
|
Table 1 The nitrogen adsorption–desorption isotherm and pore size distributions of the N-doped PC. |
The TEM and SEM images of Zn-free N-doped PC-900 and Znfree N-doped PC-900-Pd samples are shown in Fig. 1. Comparing with Fig. 1A and B,it can be clearly seen that the Pd nanoparticles were highly dispersed on the surface of Zn-free N-doped PC-900. The average size of the Pd nanoparticles is 3.6 nm. The results indicated that the high BET and the pore volume of Zn-free Ndoped PC-900 promote the dispersion of Pd. Besides,nitrogen in the composite also plays an important role to improve the dispersability of Pd nanoparticles. It also can be clearly seen from the SEM image (Fig. 1C) that some of the Pd NPs were supported on the surface of the Zn-free N-doped PC-900,and part of them inserted into the cavities of Zn-free N-doped PC-900. The palladium content in Zn-free N-doped PC-900-Pd was determined by means of ICP-AES and amounted to 4.03 wt%.

|
Download:
|
| Fig. 1.TEM images of (A) Zn-free N-doped PC-900, (B) Zn-free N-doped PC-900-Pd and SEM image (C) of Zn-free N-doped PC-900-Pd. | |
The XPS spectra (Fig. 2A) demonstrated that the Pd species in the Zn-free N-doped PC-900-Pd sample are present in the metallic state with the bond energy about 335.5 and 340.98 eV in the Pd 3d5/2 and 3d3/2 core level,and Pd 3d spectrum can be deconvoluted to four sub-peaks,containing Pd0 3d3/2 (334.7 eV),Pd0 3d5/2 (340.1 eV),Pd2+ 3d3/2 (341.2 eV) and Pd2+ 3d5/2 (336.1 eV) [35, 36]. Moreover,the high resolution nitrogen (N) 1s spectrum can be deconvoluted to two sub-peaks due to the spin orbit coupling, including pyridinic-N (398.51 eV) and graphitic-N (400.98 eV) [37, 38],which is a common characteristic for nitrogen-doped carbon materials. In general,nitrogen in a carbon texture is suitable for stabilizing highly dispersed metal nanoparticles. The nitrogen content in Zn-free N-doped PC-900-Pd was determined by means of XPS and amounted to 10 wt%.

|
Download:
|
| Fig. 2.The XPS image of Zn-free N-doped PC-900. | |
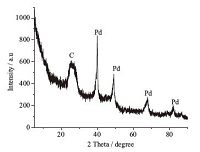
Fig. 3 displays the XRD pattern of Zn-free N-doped PC-900-Pd composite. The wide diffraction peak at 2θ= 25° can be indexed to porous carbon [39]. The well-defined peaks around 408,478,688 and 838 can be assigned to (1 1 1),(2 0 0),(2 2 0) and (3 1 1) crystal planes of Pd0 in the composite.
|
Download:
|
| Fig. 3.XRD patterns of Zn-free N-doped PC-900-Pd. C: porous carbon. | |
In order to investigate the catalytic activity of different catalysts for the C-C coupling reaction,the coupling of phenylbromide and phenylboronic acid was selected as a model reaction. As shown in Table 2,among the catalysts tested at room temperature,Zn-free N-doped PC-900-Pd was found to be the most effective catalyst since it gave the highest yield of product. Good dispersion of the active species (Pd) on the support is a key factor for the catalytic activity of the catalyst. This is mainly due to the high surface area of Zn-free N-doped PC-900,which leads to high dispersion of Pd on the surface of support. Additionally,the presence of nitrogen element in the carbon texture was also helpful to increase the dispersion of the active species.
|
|
Table 2 Suzuki–Miyaura coupling reactions catalyzed by different catalysts. |
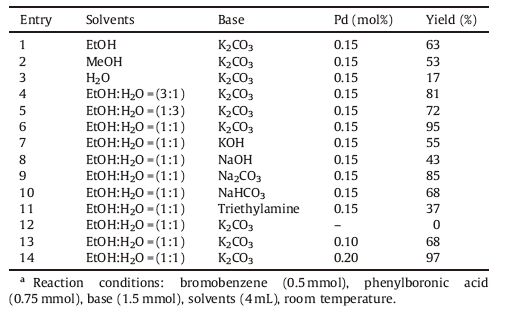
To evaluate the catalytic performance of the as-obtained catalyst and optimize the reaction conditions of the Suzuki coupling reaction,the reaction between phenylbromide and phenylboronic acid was chosen as model reaction. Initially,single solvents,such as EtOH,MeOH,and H2O,were studied. As documented in Table 3,the single solvents gave low yields for the reaction (Table 3,entries 1-3). However,when we adopted the organic/aqueous co-solvent,high yields of 72%-95% were obtained (Table 3,entries 4-6). The beneficial effect of the co-solvent may be attributed to the good solubility of the organic reactants and the inorganic base,so EtOH:H2O (1:1,v/v) was chosen as the reaction solvent for the subsequent reactions. As is well known,a base has a strong influence on Suzuki reactions. When the organic and inorganic bases,such as Et3N,K2CO3,Na2CO3,NaOH,KOH,NaHCO3, were taken into consideration for the Suzuki coupling reaction (Table 3,entries 7-11),the experiments showed that K2CO3,as a base,afforded the product in the highest yield. It was also determined that the amount of the catalyst has a great influence on the transformation (Table 3,entries 6,12-14),and the best results were obtained with 0.15 mol% of catalyst. When the amount of the catalyst was increase to 0.20 mol%,the product was obtained in a nearly quantitative yield (Table 3,entry 14). However,the yield of the reaction decreased to 68% (Table 3,entry 13) in 0.10 mol% Pd, and the coupling reaction does not proceed at all in the absence of Pd nanoparticles (Table 3,entry 12).
|
|
Table 3 Optimization of the reaction conditions for the Suzuki reaction of bromobenzene with phenylboronic acid.a |
After optimizing the reaction conditions,the catalytic activity of Zn-free N-doped PC-900-Pd for the Suzuki-Miyaura reaction was explored with respect to various aryl halides and phenylboronic acids. It was found that the catalyst showed a high reactivity and selectivity for aryl bromides with an electron-withdrawing group and electron-donating group. As shown in Table 4,the coupling reactions could be proceeded well and the reactions could be completed in 0.5-2 h at room temperature with good to excellent yields. Typically,compared with the electron-donating groups on aryl bromides,such as -CH3,-OCH3 (Table 4,entries 2-3,8-9),the electron-withdrawing groups,such as -COCH3,-OH (Table 4, entries 4-7,11-13),have higher reaction activity. To test the feasibility of the aforementioned protocol for challenging substrates, several aryl chlorides with aryl boronic acids were employed and the reaction time was increased to 8 h. Unfortunately, the catalytic system was less effective for the reaction of aryl chlorides (Table 4,entries 14 and 15) because of the strong strength of the C-Cl bond,whose bond dissociation energy was 96 kcal/mol [40].
|
|
Table 4 Suzuki–Miyaura coupling of aryl boronic acids and aryl halides catalyzed by N-doped PC-900-Pd.a |
The recycling and reusability of a catalyst is very important for industrial uses. Thus,the reusability of Zn-free N-doped PC-900-Pd was studied in the Suzuki-Miyaura cross-coupling of bromobenzene with phenylboronic acid under the optimal reaction conditions. As can be seen in Fig. 4,after being recycled for six successive runs,the catalyst still exhibits an excellent activity. Good reusability is mainly attributed to the high specific surface area and pore volume of Zn-free N-doped PC-900. Additionally,the nitrogen element in carbon texture also plays an important role for stabilizing highly dispersed Pd nanoparticles. Therefore,the Znfree N-doped PC-900 catalyst exhibits both excellent catalytic activity and good reusability.

|
Download:
|
| Fig. 4.Recyclability of the N-doped PC-Pd for the Suzuki–Miyaura reaction. | |
In summary,we have demonstrated the fabrication of nitrogendoped carbon by a facile,low-cost and readily reproducible approach using ZIF-8 as precursor,and the prepared N-doped PC was used as the carrier material for palladium nanoparticle. The resultant Zn-free N-doped PC-900-Pd offers high surface area and exhibits a significant activity toward the Suzuki coupling reaction between aryl halide and aryl boronic acid,even when the reaction was carried out at room temperature for a relatively short time. Moreover,good yields were obtained even after the Zn-free N-doped PC-900-Pd catalyst was reused six times. The present research might highlight the development of high catalytic activity heterogeneous catalysts by using MOF-derived porous carbon as hosts for ultrafine metal nanoparticles.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 31171698,31471643),the Innovation Research Program of Department of Education of Hebei for Hebei Provincial Universities (No. LJRC009),Natural Science Foundation of Hebei Province (No. B2015204003) and the Natural Science Foundation of Agricultural University of Hebei (Nos. LG201404,ZD201506).
| [1] | M.B. Thathagar, J. Beckers, G. Rothenberg, Copper-catalyzed suzuki cross-coupling using mixed nanocluster catalysts, J. Am. Chem. Soc. 124(2002) 11858-11859. |
| [2] | N.Z. Shang, C. Feng, H.Y. Zhang, et al., Suzuki-Miyaura reaction catalyzed by graphene oxide supported palladium nanoparticles, Catal. Commun. 40(2013) 111-115. |
| [3] | Y.Y. Ma, X.B. Ma, Q. Wang, J.Q. Zhou, Homogenization of inorganic materialsupported palladium catalysts in Suzuki coupling reaction at room temperature, Catal. Sci. Technol. 2(2012) 1879. |
| [4] | A.R. Siamaki, A.E.R.S. Khder, V. Abdelsayed, M.S. El-Shall, B.F. Gupton, Microwaveassisted synthesis of palladium nanoparticles supported on graphene:a highly active and recyclable catalyst for carbon-carbon cross-coupling reactions, J. Catal. 279(2011) 1-11. |
| [5] | M. Amini, A. Tarassoli, S. Yousefi, et al., Suzuki-Miyaura cross-coupling reactions in water using in situ generated palladium(Ⅱ)-phosphazane complexes, Chin. Chem. Lett. 25(2014) 166-168. |
| [6] | H. Cheng, Q.Y. Wu, F. Han, G.F. Yang, Efficient synthesis of 4-substituted pyrazole via microwave-promoted Suzuki cross-coupling reaction, Chin. Chem. Lett. 25(2014) 705-709. |
| [7] | C.M. Chen, L.Y. Wei, X.H. Guo, S.X. Guo, G. Yan, Investigation of heavy oil refinery wastewater treatment by integrated ozone and activated carbon-supported manganese oxides, Fuel Process. Technol. 124(2014) 165-173. |
| [8] | L.J. Wang, Q. Han, D.C. Li, et al., Comparisons of Pt catalysts supported on active carbon, carbon molecular sieve, carbon nanotubes and graphite for HI decomposition at different temperature, J. Hydrogen Energy 38(2013) 109-116. |
| [9] | H. Veisi, R. Masti, D. Kordestani, M. Safaei, O. Sahin, Functionalization of fullerene(C60) with metformine to immobilized palladium as a novel heterogeneous and reusable nanocatalyst in the Suzuki-Miyaura coupling reaction at room temperature, J. Mol. Catal. A:Chem. 385(2014) 61-67. |
| [10] | H. Veisi, A. Khazaei, M. Safaei, D. Kordestani, Synthesis of biguanide-functionalized single-walled carbon nanotubes(SWCNTs) hybrid materials to immobilized palladium as new recyclable heterogeneous nanocatalyst for Suzuki-Miyaura coupling reaction, J. Mol. Catal. A:Chem. 382(2014) 106-113. |
| [11] | J.F. Hu, Y.P. Wang, M. Han, et al., A facile preparation of palladium nanoparticles supported on magnetite/s-graphene and their catalytic application in Suzuki-Miyaura reaction, Catal. Sci. Technol. 2(2012) 2332-2340. |
| [12] | Q. Xu, J.K. Sun, Functional materials derived from open framework templates/precursors:synthesis and applications, Energy Environ. 7(2014) 2071-2100. |
| [13] | M. Bakherad, S. Jajarmi, A dithizone-functionalized polystyrene resin-supported Pd(Ⅱ) complex as an effective catalyst for Suzuki, Heck, and copper-free Sonogashira reactions under aerobic conditions in water, J. Mol. Catal. A:Chem. 370(2013) 152-159. |
| [14] | X. Xu, Y. Li, Y.T. Gong, et al., Synthesis of palladium nanoparticles supported on mesoporous N-doped carbon and their catalytic ability for biofuel upgrade, J. Am. Chem. Soc. 134(2012) 16987-16990. |
| [15] | M. Li, X. Xu, Y. Gong, et al., Ultrafinely dispersed Pd nanoparticles on a CN@MgO hybrid as a bifunctional catalyst for upgrading bioderived compounds, Green Chem. 16(2014) 4371. |
| [16] | Y. Wang, J. Yao, H.R. Li, D.S. Su, M. Antonietti, Highly selective hydrogenation of phenol and derivatives over a Pd@carbon nitride catalyst in aqueous media, J. Am. Chem. Soc. 133(2011) 2362-2365. |
| [17] | M. Hu, J. Reboul, S. Furukawa, et al., Direct carbonization of Al-based porous coordination polymer for synthesis of nanoporous carbon, J. Am. Chem. Soc. 134(2012) 2864-2867. |
| [18] | L. Zhang, Z. Su, F. Jiang, et al., Highly graphitized nitrogen-doped porous carbon nanopolyhedra derived from ZIF-8 nanocrystals as efficient electrocatalysts for oxygen reduction reactions, Nanoscale 6(2014) 6590-6602. |
| [19] | X. Zhao, H. Zhao, T. Zhang, et al., One-step synthesis of nitrogen-doped microporous carbon materials as metal-free electrocatalysts for oxygen reduction reaction, J. Mater. Chem. A 2(2014) 11666. |
| [20] | W. Chaikittisilp, M. Hu, H.J. Wang, et al., Nanoporous carbons through direct carbonization of a zeolitic imidazolate framework for supercapacitor electrodes, Chem. Commun. 48(2012) 7259-7261. |
| [21] | X.L. Yan, X.J. Li, Z.F. Yan, S. Komarneni, Porous carbons prepared by direct carbonization of MOFs for supercapacitors, Appl. Surf. Sci. 308(2014) 306-310. |
| [22] | F. Afsahi, H.V. Thang, S. Mikhailenko, S. Kaliaguine, Electrocatalyst synthesized from metal organic frameworks, J. Power Sources 239(2013) 415-423. |
| [23] | W. Chaikittisilp, K. Ariga, Y. Yamauchi, A new family of carbon materials:synthesis of MOF-derived nanoporous carbons and their promising applications, J. Mater. Chem. A 1(2013) 14-19. |
| [24] | H.L. Jiang, B. Liu, Y.Q. Lan, et al., From metal-organic framework to nanoporous carbon:toward a very high surface area and hydrogen uptake, J. Am. Chem. Soc. 133(2011) 11854-11857. |
| [25] | J. Kim, N.D. McNamara, T.H. Her, J.C. Hicks, Carbothermal reduction of Ti-modified IRMOF-3:an adaptable synthetic method to support catalytic nanoparticles on carbon, ACS Appl. Mater. Interfaces 5(2013) 11479-11487. |
| [26] | G. Srinivas, V. Krungleviciute, Z.X. Guo, T. Yildirim, T. Yildirim, Exceptional CO2 capture in a hierarchically porous carbon with simultaneous high surface area and pore volume, Energy Environ. 7(2014) 335-342. |
| [27] | T. Ahnfeldt, N. Guillou, D. Gunzelmann, et al.,[Al4(OH)2(OCH3)4(H2Nbdc)3]·xH2O:a 12-connected porous metal-organic framework with an unprecedented aluminum-containing brick, Angew. Chem. Int. Ed. Engl. 48(2009) 5163-5166. |
| [28] | Y.Y. Lu, W.W. Zhan, Y. He, et al., MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties, ACS Appl. Mater. Interfaces 6(2014) 4186-4195. |
| [29] | S.J. Yang, T. Kin, J.H. Im, et al., MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity, Chem. Mater. 24(2012) 464-470. |
| [30] | S.J. Yang, T. Kim, K. Lee, et al., Solvent evaporation mediated preparation of hierarchically porous metal organic framework-derived carbon with controllable and accessible large-scale porosity, Carbon 71(2014) 294-302. |
| [31] | H.B. Aiyappa, P. Pachfule, R. Banerjee, S. Kurungot, Porous carbons from nonporous MOFs:influence of ligand characteristics on intrinsic properties of end carbon, Cryst. Growth Des. 13(2013) 4195-4199. |
| [32] | N.Z. Shang, S.T. Gao, X. Zhou, et al. Palladium nanoparticles encapsulated inside the pores of a metal-organic framework as a highly active catalyst for carbon-carbon cross-coupling RSC Adv. 4(2014) 54487-54493. |
| [33] | L. Zhang, C. Feng, S.T. Gao, Z. Wang, C. Wang, Palladium nanoparticles supported on metal organic framework derived N-decorated nanoporous carbon as an efficient catalyst for the Suzuki coupling reaction, Catal. Commun. 61(2015) 21-25. |
| [34] | X. Liu, L. Zhou, Y. Zhao, et al., Hollow, spherical nitrogen-rich porous carbon shells obtained from a porous organic framework for the supercapacitor, ACS Appl. Mater. Interfaces 5(2013) 10280-10287. |
| [35] | S.Y. Lin, X. Yang, L.Y. Yang, R.X. Zhou, Three-way catalytic performance of Pd/Ce0.67Zr0.33O2-Al2O3 catalysts:role of the different Pd precursors, Appl. Surf. Sci. 327(2015) 335-343. |
| [36] | A. Maksic, Z. Rakocevic, M. Smiljanic, M. Nenadovic, S. Strbac, Methanol oxidation on Pd/Pt(poly) in alkaline solution, J. Power Sources 273(2015) 724-734. |
| [37] | E. Negro, A.H.A.M. Videla, V. Baglio, et al., Fe-N supported on graphitic carbon nano-networks grown from cobalt as oxygen reduction catalysts for low-temperature fuel cells, Appl. Catal. B:Environ. 166-167(2015) 75-83. |
| [38] | S.Z. Wu, Y.X. Yu, W.D. Zhang, Processing graphitic carbon nitride for improved photocatalytic activity, Mat. Sci. Semicon. Proc. 24(2014) 15-20. |
| [39] | B. Liu, T. Akita, Q. Xu, Metal-organic framework as a template for porous carbon synthesis, J. Am. Chem. Soc. 130(2008) 5390-5391. |
| [40] | M.R. Feyyaz Durap, M. Aydemir, S. Ö zkar, Room temperature aerobic Suzuki cross-coupling reactions in DMF/water mixture using zeolite confined palladium(0) nanoclusters as efficient and recyclable catalyst, Appl. Catal. A:Gen. 382(2010) 339-344. |
 2016, Vol.27
2016, Vol.27