ILs have received much attention in many areas of chemistry and industry due to their potential as "green" recyclable alternatives to traditional organic solvents [1, 2]. Prior to the year 2000,ionic liquids had been successfully used in synthesis of polymers. The related reports mainly focused on Ziegler-Natta polymerization of ethylene [3, 4, 5, 6] and metathesis polymerization of acyclic dienes [7, 8]. Those rather preliminary studies revealed that it is possible to conduct different polymerization processes in ILs. Afterwards,the application of ILs in synthesis of polymer spread to several new types of polymerization processes,including conventional free radical polymerization [9],living/controlling radical polymerizations [10, 11],reversible addition-fragmentation transfer (RAFT) [12],as well as in coordination polymerizations [13]. As a result,several advantages of ILs in polymer synthesis were shown [14],including increase of the propagation rate constant and decrease of the termination rate constant,resulting in better control in ATRP [8].
Besides exhibiting many advantages as the solvent,ILs also play an important catalytic role in polymer synthesis. A few reports have found that the polymerization rate using [Bmim][PF]6 ionic liquid in styrene and methyl methacrylate system was 10 times as fast as in benzene [15].
Poly(vinyl butyral) (PVB) is a member of the class of poly(vinyl acetal) resins. Owing to its several advantages,such as high adhesion to glass,toughness,light stability,clarity as well as excellent mechanical strength and impact resistance,PVB has many desirable applications [16, 17]. Synthesis of PVB by the precipitation method is used in industry production for its low cost and simple operation. However,this method is achieved by acetalization of polyvinyl alcohol (PVA) and butyraldehyde (BA) in the presence of the strong inorganic acid,which produces a lot of waste acid water and results in the corrosion of equipment. The synthesis of PVB using ILs instead of inorganic acid has never been developed as of yet.
The present work was to develop an environment-friendly synthesis of PVB by using IL catalyst. The characterizations of structure and properties of PVB were studies.
2. Experimental 2.1. Preparation of ionic liquid[HMIM]+HSO4- ionic liquid was prepared according to literature procedures [18]. N-Methylimidazole (Sinopharm Chemical Reagent Co.,Ltd.) was used as the starting material for preparation of [HMIM]+HSO4-. Firstly,a certain amount of N-methylimidazole was added into the flask,and then a few drops of H2SO4 solution was added with vigorous stirring at 0 ℃ for 4 h. Finally,the resulting product was dried under vacuum at 50 ℃ for 12 h.
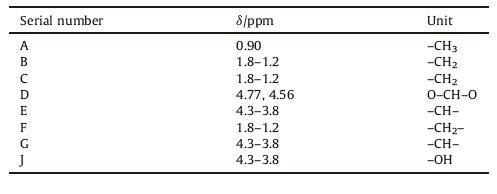
2.2. Preparation of PVBPolyvinyl alcohol (PVA,Sinopharm Chemical Reagent Co.,Ltd.) was used as the starting material for preparation of PVB. Firstly,an amount of PVA was dissolved in deionized water with vigorous stirring at 95 ℃ for 2 h. The flask was immersed in a water bath. At 50 ℃,0.5 g of lauryl sodium sulfate was added into the above solution under stirring for 30 min. Then,n-butyraldehyde (98.5%, Sinopharm Chemical Reagent Co.,Ltd.) (mBA:mPVA = 0.6) was added into the above solution with vigorous stirring for 30 min at 30 ℃. After cooling to below 20 ℃,[HMIM]+HSO4- ionic liquid was added into the solution with vigorous stirring for 4 h. The resulting mixture was continually stirred at 7-15 ℃ for 4 h,and then at 4 ℃ for 4 h. The as-obtained acetal product was washed several times with deionized water and finally dried under the freeze drying machine. The sample was denoted self-made PVB. The synthesis route was shown in Scheme 1.
|
Download:
|
| Scheme 1.Synthesis of PVB. | |
After the completion of above reaction,the PVB product and the solution containing ILs were successfully separated by vacuum extraction filtration. The separated solution as catalyst and solvent was applied to new PVB synthesis without adding fresh IL. In the recycling process,only an amount of deionized water was supplemented in order to recover the solvent loss.
2.4. CharacterizationPolymer functional groups were analyzed by infrared spectroscopy (Cary 610/670 Micro infrared spectrometer) in the range of 4000-400 cm-1 by using KBr pellets. The configuration structure of polymers was detected by NMR spectroscopy (Bruker AV-400NMR spectrometer). The DSC analysis of polymer was recorded by DSC 8500. The molecular weight of polymer was analyzed by Agilent GPC. The viscosity was measured by SNB-1A Digital Viscometer.
2.5. Measurement for the acetalization degreeThe measurement of the acetalization degree: firstly,1 g of PVB was added into 50 mL 95% alcohol under vigorous stirring for 2 h. Then 25 mL 7.5% hydroxylamine was added into the above solution under vigorous stirred for another 2 h and cooled to the room temperature. Finally,bromophenol blue was added, and the solution was titrated to purple with a standard solution of sodium hydroxide. The vacuity contrast group also was done [19].
The calculation formula:
B=0.142×(V-V0×c/m)
V - the amount of sodium hydroxide solution consumed samples of PVB,mL;
V0 - the amount of sodium hydroxide solution consumed samples of the vacuity contrast group,mL;
c - the concentration of sodium hydroxide,mol/L; m - the mass of PVB.
3. Results and discussionThe self-made PVB and the commercial PVB exhibit similar main peaks in FT-IR spectra as shown in Fig. 1. For the self-made PVB,the peaks at 2873 cm-1,2961 cm-1 are ascribed to the stretching vibration of saturated -CH,-CH2 and -CH3. The peaks at 1439 cm-1,1382 cm-1 and 1348 cm-1 are assigned to C-H bending vibration. The peaks at 1133 cm-1 and 1050 cm-1 are assumed to C-O-C-O-C stretching vibrations of five-membered cyclic acetal group and hexatomic cyclic acetal group,which confirms the occurrence of acetalization reaction between polyvinyl alcohol and n-butyraldehyde. The peak at 1730 cm-1 relates to C=O stretching vibration of acetate group. The peaks at 997 cm-1 and 1242 cm-1 are ascribed to C-O-C stretching vibration of acetate group [20, 21]. The peak at 3440 cm-1 is assigned to -OH asymmetric stretching of polyvinyl alcohol,which shows a reduction in magnitude compared with the commercial PVB samples.

|
Download:
|
| Fig. 1.FT-IR spectra of PVA, commercial PVB and PVB obtained by using [HMIM]+HSO4- IL catalyst. | |
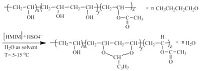
Fig. 2 gives the 1H NMR spectra of PVB by obtained using [HMIM]+HSO4- IL catalyst and Table 1 lists theNMR data. As can be proved,the molecular structure of pure PVB is distinctly presented in spectra and table. Among all the polyvinyl acetals,NMR data are available only for polyvinyl butyral and polyvinyl form. These results are consistent with the report [22]. This illustrates that the synthesis of PVB by using ionic liquids instead of inorganic acid is feasible.
|
Download:
|
| Fig. 2.1H NMR spectra of PVB obtained by using [HMIM]+HSO4- IL catalyst. | |
|
|
Table 1 The 1H NMR data of PVB. |
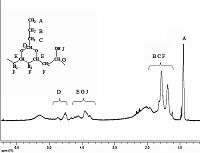
Fig. 3 shows the DSC analysis of the commercial PVB and the self-made PVB. Compared to the commercial PVB,there is an endothermic peak at 75.2 ℃ in the curve of the self-made PVB, which should be the melting peak of PVB. Most interestingly,the melting peak of the self-made PVB appears shifted toward high temperature,increasing about 2 ℃. This should be ascribed to the difference of molecular structure of between the commercial PVB and the self-made PVB.
|
Download:
|
| Fig. 3.DSC analysis of commercial PVB and self-made PVB. | |
Fig. 4 displays the SEM images of self-made PVB and commercial PVB. It can be observed that the particle size of selfmade PVB is smaller,and the regularity of particles is better than those of commercial PVB.

|
Download:
|
| Fig. 4.SEM images of PVB (a, commercial PVB) and (b, self-made PVB). | |
Fig. 5 shows the catalytic activity of [HMIM]+HSO4- IL catalyst for synthesis of PVB. As can be seen,the acetalization degree of PVB has been significantly affected by the concentration of PVA in water. When the concentration PVA in water is 8%,the acetalization degree of PVB attains the maximum value of 72%.

|
Download:
|
| Fig. 5.Acetalization degree of PVB as function of the concentration of PVA in water solution by using [HMIM]+HSO4- IL catalyst. | |
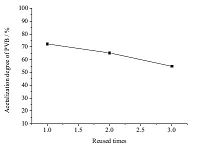
Fig. 6 shows the effect of the reuse of [HMIM]+HSO4- IL solution on the acetalization degree of PVB. As the number of times the IL solution is reused increases,the acetalization degree decreases. The reason should be ascribed to the loss of IL in the reused solution during the separation process of PVB product and IL solution.
|
Download:
|
| Fig. 6.Acetalization degree of PVB as function of the reused times of [HMIM]+HSO4-. | |
Table 2 lists the properties of PVA,the commercial PVB and selfmade PVB obtained by [HMIM]+HSO4- IL catalyst. It is found that the molecular weight of PVA via acetalization significantly increases from 8 w to 28 w,which is attributed to the result of PVA converted into PVB product. The self-made PVB obtained by using [HMIM]+HSO4- IL catalyst has a larger molecular weight, higher viscosity,and higher acetalization degree than the commercial PVB. This suggests that the synthesis approach of PVB by using [HMIM]+HSO4- IL catalyst is desirable and promising for environment-friendly production.
|
|
Table 2 The properties of the commercial PVB and self-made PVB. |
Based on above results,the reaction mechanism of PVB synthesis by using [HMIM]+HSO4- IL catalyst was represented in Scheme 2. It can be inferred that [HMIM]+HSO4- catalyst through ionization in water solution released out [H+] ion that acts as the same catalyzed mechanism as inorganic acid.

|
Download:
|
| Scheme 2.The reaction mechanism of PVB synthesis by using [HMIM]+HSO4- IL catalyst. | |
4. Conclusion This paper developed an environmentally friendly method for synthesis of polyvinyl butyral (PVB) by using [HMIM]+HSO4- IL catalyst. The characterizations verified the structure of PVB obtained by using IL catalyst. The PVB obtained by using [HMIM]+HSO4- IL catalyst exhibited better properties than the commercial PVB.
AcknowledgmentsThis work was supported by the Talent Introduction Fund of Yangzhou University,Jiangsu Social Development Project (No. BE2014613) and Six Talent Peaks of Jiangsu Province (No. 2014-XCL-013). The authors also acknowledge the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. The data of this paper originated from the Test center of Yangzhou University.
| [1] | F. Shirini, A. Yahyazadeh, K. Mohammadi, One-pot synthesis of various xanthene derivatives using ionic liquid 1,3-disulfonic acid imidazolium hydrogen sulfate as an efficient and reusable catalyst under solvent-free conditions, Chin. Chem. Lett. 25(2014) 341-347. |
| [2] | H. Singh, S. Kumari, J.M. Khurana, A new green approach for the synthesis of 12-aryl-8, 9, 10, 12-tetrahydrobenzo[a]xanthene-11-one derivatives using task specific acidic ionic liquid[NMP]H2PO4, Chin. Chem. Lett. 25(2014) 1336-1340. |
| [3] | R.T. Carlin, R.A. Osteryoung, J.S. Wilkes, J. Rovang, Studies of titanium(IV) chloride in a strongly Lewis acidic molten salt:electrochemistry and titanium NMR and electronic spectroscopy, Inorg. Chem. 29(1990) 3003-3009. |
| [4] | R.T. Carlin, J.S. Wilkes, Complexation of Cp2MCl2 in a chloroaluminate molten salt:relevance to homogeneous Ziegler-Natta catalysis, J. Mol. Catal. 63(1990) 125-129. |
| [5] | M.F. Pinheiro, R.S. Mauler, R.F. de Souza, Biphasic ethylene polymerization with a diiminenickel catalyst, Macromol. Rapid Commun. 22(2001) 425-428. |
| [6] | P. Wasserscheid, C.M. Gordon, C. Hilgers, M.J. Muldoon, I.R. Dunkin, Ionic liquids:polar, but weakly coordinating solvents for the first biphasic oligomerisation of ethene to higher a-olefins with cationic Ni complexes, Chem. Commun. 13(2001) 1186-1187. |
| [7] | A. Fürstner, Olefin metathesis and beyond, Angew. Chem. Int. Ed. 39(2000) 3012-3043. |
| [8] | P. Kubisa, Application of ionic liquids as solvents for polymerization processes, Prog. Polym. Sci. 29(2004) 3-12. |
| [9] | Y.S. Vygodskii, D.A. Sapozhnikov, A.S. Shaplov, et al., New ionic liquids with hydrolytically stable anions as alternatives to hexafluorophosphate and tetrafluoroborate salts in the free radical polymerization and preparation of ionconducting composites, Polym. J. 43(2011) 126-135. |
| [10] | P. Kubisa, Ionic liquids as solvents for polymerization processes-progress and challenges, Prog. Polym. Sci. 34(2009) 1333-1347. |
| [11] | Z.Z. Chen, L.H. Qiu, J.M. Lu, F. Yan, J. Texter, Atom transfer radical polymerization in ionic liquid-based microemulsions, Polym. Prepr. 51(2010) 559-560. |
| [12] | C.X. Lin, H.Y. Zhan, M.H. Liu, et al., RAFT synthesis of cellulose-g-polymethylmethacrylate copolymer in an ionic liquid, J. Appl. Polym. Sci. 127(2013) 4840-4849. |
| [13] | R. Vijayaraghavan, D.R. MacFarlane, Living cationic polymerisation of styrene in an ionic liquid, Chem. Commun. 6(2004) 700-701. |
| [14] | M.J. Shi, S.Z. Kou, B.S. Shen, et al., Improving the performance of all-solid-state supercapacitors by modifying ionic liquid gel electrolytes with graphene nanosheets prepared by arc-discharge, Chin. Chem. Lett. 25(2014) 859-864. |
| [15] | K.L. Hong, H.W. Zhang, J.W. Mays, et al., Conventional free radical polymerization inroom temperature ionic liquids:agreen approachtocommoditypolymerswith practical advantages, Chem. Commun. 13(2002) 1368-1369. |
| [16] | M. Chanda, S.K. Roy, Plastics Technology Handbook, 4th ed., CRC Press, 2006. |
| [17] | A. Kraft, M. Rottmann, Properties, performance and current status of the laminated electrochromic glass of Gesimat, Sol. Energy Mater. Sol. Cells 93(2009) 2088-2092. |
| [18] | Q.B. Zhang, Y.X. Hua, Effects of[HMIM]HSO4 and[OMIM]HSO4 on the electrodeposition of zinc from sulfate electrolytes, J. Appl. Electrochem. 39(2009) 1185-1192. |
| [19] | B. Yang, R.G. Liu, J.J. Huang, H. Sun, Reverse dissolution as a route in the synthesis of poly(vinyl butyral) with high butyral contents, Ind. Eng. Chem. Res. 52(2013) 7425-7431. |
| [20] | A. Eceiza, K. de la Caba, G. Kortaberria, et al., Influence of molecular weight and chemical structure of soft segment in reaction kinetics of polycarbonate diols with 4,4'-diphenylmethane diisocyanate, Eur. Polym. J. 41(2005) 3051-3059. |
| [21] | S. Subramani, Y.-J. Park, Y.-S. Lee, J.-H. Kim, New development of polyurethane dispersion derived from blocked aromatic diisocyanate, Prog. Org. Coat. 48(2003) 71-79. |
| [22] | Y.A. Kurskii, L.N. Beloded, N.K. Kobyakova, et al., A GLC and NMR analysis of the composition of polyvinyl butyral furfural acetal, Russ. J. Appl. Chem. 84(2011) 1914-1919. |
 2016, Vol.27
2016, Vol.27