DNA delivery,protection,and purification are important during gene therapy of a wide range of diseases; as such,developing efficient methods to purify DNA is a desirable endeavor for many researchers. There are two main methods for extracting nucleic acid. One is the fluid-phase method with organic solvents [1], which often involve chemicals that are toxic to humans and the environment. Another method is solid-phase extraction,which has been widely used in DNA separation,purification and enrichment [2, 3, 4]. The reported solid agents include silica [3] and its derivatives [5],magnetic beads [6],polymer [7],and magnetic silica particles [8]. Compared with other materials,ordered mesoporous silica materials,such as MCM-41,SBA-15,and mesoporous silica nanoparticles,have been widely studied for adsorption of biological samples because of their advantageous structural properties,which include large surface areas and pore volumes,good biocompatibility,and potential for specific functionalization of various materials [9, 10, 11].
The DNA-silica interaction is electrostatically unfavorable,since bothDNAand silica substrate exhibit negative surface chargesunder usual experimental conditions [12]. To adsorbDNA,the electrostatic repulsion between DNA and absorbents needs to be reduced. Therefore,many developments in modifying mesoporous silica materials have sought to increase their adsorption capacity and improve their release properties. Choi et al. conducted amino treatment with mono-,di-,and tri-amino functional groups on the inner surfaces of a mesoporous silica material; results clearly demonstrated that this mesoporous material could effectively bind DNA by introducing electrostatic interactions [2]. Jiang et al. provided an alternative approach for highly efficient DNA extraction with Fe3+-immobilized silica particles by building a salt bridge [13]. Gu’s group [14] utilized chaotropic salt solution to enhance DNA adsorption into magnetic mesoporous silica nanoparticles by shielding negatively charged species. However,the residual chaotropic salt afterpurification could inhibit down-stream processes [12]. An electrical switch technology for modulating DNA adsorption on silica beads was reported [15]. The pH change altered the amount of DNA molecules adsorbed. Much effort has been exerted to drive DNA adsorption and desorption on silica particles. MCM-41 and modifiedMCM-41molecular sieves have been widely studied because they are promising as adsorbents for removal of environmental pollutants [16],catalysts,and catalyst supports [17, 18]. However,few investigations have reported metal ionfunctionalized MCM-41 as an absorbent for DNA. In this study,a novel method for DNA enrichment is provided.
This paper presents the fabrication of mesoporous MCM-41 adsorbents that had been modified by amino groups,phosphonate groups,and metal cations,and all products were characterized by Fourier transform infrared (FTIR) spectroscopy,thermogravimetric analysis (TGA),and nitrogen adsorption-desorption isotherms. The adsorption and desorption behaviors of DNA on functionalized MCM-41 were investigated by varying the experimental conditions, including adsorption time,amount of adsorbent used,and eluent pH. A relevant adsorption mechanism was also discussed.
2. Experimental 2.1. Synthesis of metal ion-modified MCM-41The procedures for the preparation of metal ion-modified MCM- 41 included two stages. First,phosphonate-modified MCM-41 was fabricated according to previously reported procedures [19]. MCM- 41 (0.5 g) was dispersed in toluene (25 mL),after which 3- aminopropyl-triethoxysilane (1.5 mL) was added to the solution. The mixture was stirred and refluxed for 12 h at 110 ℃ under nitrogen gas protection. After filtering andwashing,the productwas dried at 60 ℃ in vacuum,and the aminopropyl-functionalized MCM-41 (labeled NH2-MCM-41) was obtained. NH2-MCM-41 (0.2 g) was dispersed in toluene (15mL),after which pyridine (0.5mL) and POCl3 (0.5mL) were added to the solution. Themixture solution was stirred and reacted at room temperature for 18 h to obtain phosphonate-modifiedMCM-41. The product wasmarked as PO3--MCM-41 after washing and drying. Second,phosphonatemodified MCM-41 (0.1 g) and Al(NO3)3 (0.2 mol L-1) aqueous solution (20 mL) were mixed and stirred for 24 h at room temperature. The sample was then filtered,washed three times with distilledwater,andfinallydriedin vacuumat60 ℃for24 h.The obtained product was designated as Al3+-MCM-41 and stored at 4 ℃. To immobilize other ions (La3+ and Zn2+),the same procedure was carried out,and the generated metal ion-terminated MCM-41 samples were labeled La3+-MCM-41 and Zn2+-MCM-41,respectively. Here,a large excess of metal ions (Al3+,La3+,and Zn2+) was added to ensure that a consistent amount of metal ions was loaded onto the MCM-41 surface [20].
2.2. DNA adsorption and desorption experimentsDNA adsorption experiments were carried out to evaluate the adsorption capacity of the metal ion-functionalized MCM-41. In each experiment,a certain amount of adsorbent was mixed with 5 mL of the DNA standard solution (10-4gmL-1) under ultrasound for a certain period of time. The DNA concentration in the supernatant after centrifugation was measured with a UV-vis spectrophotometer. The effects of various factors,such as adsorbent amount and adsorption time were analyzed. The adsorption efficiency is generally expressed in Eq. (1):
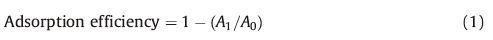
All samples were allowed to undergo DNA adsorption prior to a desorption study at optimal conditions. DNA desorption was conducted using 5 mL of NaCl solution at concentrations ranging from 0.5 mol L-1 to 6 mol L-1 in ultrasound conditions. DNA absorbance in the supernatant after elution and centrifugation was measured on a UV-vis spectrophotometer at 258 nm. In particular, the effects of different desorption conditions,including pH,elution concentration,and elution time,on recovery efficiency were evaluated. The recovery ratio is calculated using Eq. (2):

FTIR spectra were collected using a Nicolet 5700 spectrometer at room temperature in the spectral range of 400-4000 cm-1. TGA was performed on a Q100 thermo-gravimetric analyzer by heating the samples at 10 ℃/min under N2 from room temperature to 800 ℃. Nitrogen adsorption-desorption isotherms were obtained using a Micromeritics ASAP 2020 analyzer. Specific surface areas were calculated using the BET method. Pore diameter was deduced by the BJH method. ICP (inductively coupled plasma,optima 5300DV) was used to measure the metal ions content. UV-vis absorption spectral studies were carried out on a Cintra 10 UV-vis spectrophotometer.
2.4. Data analysisDuring the experiments,Langmuir and Freundlich adsorption isotherms were used to evaluate the adsorption properties.
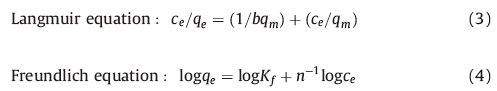
3.1. Characterization of the functionalized MCM-41
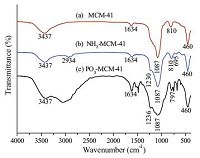
Fig. 1 shows the FTIR spectra of MCM-41,NH2-MCM-41,and PO3--MCM-41. In Fig. 1a,the broad band at 3437 cm-1 is attributed to the stretching vibration ofO-Hgroups (Si-OH). Absorption bands at 1087,810,and 460 cm-1 are ascribed to Si-O-Si asymmetric and symmetric stretching vibrations and bending vibration; these signals are the characteristic absorption peaks ofMCM[21]. Similar peaks were also found in the functionalized MCM-41 adsorbents. Fig. 1b shows two new peaks at 2934 and 1230 cm-1,which are related to -CH2 and C-N stretching vibrations,respectively [22]. At 695 cm-1,aweak peak attributedto the bending vibrations ofN-His observed [18]. These results indicate the successful decoration of the amino groups onto MCM-41. Phosphonate-modified MCM-41 was fabricated according to the literature [19]. Fig. 1c illustrates that the adsorption peak of phosphonate groups cannot be clearly observed in the range of 800-1200 cm-1 because signals in this region may overlap with those of Si-O-Si vibrations [13].
|
Download:
|
| Fig. 1.FTIR spectra of (a) MCM-41, (b) NH2-MCM-41, and (c) PO3--MCM-41. | |
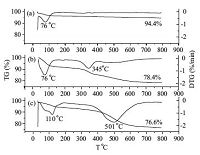
Fig. 2 presents the TG and DTG curves of MCM-41 before and after surface modification. In pure MCM-41,weight loss below 100 ℃ corresponds to the loss of adsorbed water [23]. Two distinct weight loss regions were observed in the TG curve,and two peaks were found in the DTG curve,as shown in Fig. 2b. In the first region from room temperature to 150 ℃,weight loss is attributed to desorption of adsorbed water. In the second region from 200 ℃ to 600 ℃,weight loss may be attributed to decomposition of aminopropyl groups incorporated into NH2-MCM-41 [24, 25]. In the PO3--MCM-41 sample,a peak was observed at approximately 110 ℃; this peak is likely caused by desorption of adsorbed water. Another peak observed at 501 ℃ may be due to decomposition of phosphonate groups. The combined results of FTIR spectroscopy and TGA prove that phosphonate-modified MCM-41 was successfully fabricated.
|
Download:
|
| Fig. 2.TGA and DTG curves of (a) MCM-41, (b) NH2-MCM-41, and (c) PO3--MCM-41. | |
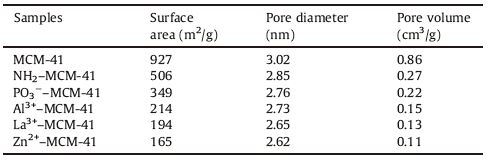
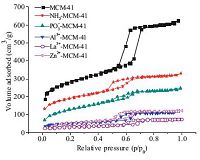
Fig. 3 shows the N2 adsorption-desorption isotherms of bare and functionalized MCM-41; the textural parameters of these samples are also listed in Table 1. A type IV isotherm with H1 hysteresis loop,representing the characteristic adsorption curve of mesoporous materials [26],was clearly seen in the parent MCM- 41. The capillary condensation of nitrogen within the mesopores appeared in the range 0.5 < P/P0 < 0.7. NH2-MCM-41 and PO3-- MCM-41 samples showed type IV isotherms,and their N2 absorption capacity decreased obviously compared with that of pure MCM-41. As shown in Table 1,a decrease in surface area,pore diameter,and pore volume was observed in the NH2-MCM-41 and PO3--MCM-41 samples compared with the parent MCM-41. These results suggest successful modification of the molecular sieve with organic functional groups. When Al3+,La3+,and Zn2+ were coordinated with the PO3--MCM-41 sample,further reductions in structural parameters were observed. The results of the N2 adsorption-desorption isotherms indicate no significant change in the mesostructure of MCM-41 during functionalization. ICP analysis showed that the loadings of Al3+,La3+,and Zn2+ on PO3--MCM-41 samples were 4.31,17.79 and 9.08 mg/g,respectively.
|
Download:
|
| Fig. 3.Nitrogen adsorption-desorption isotherms of bare and modified MCM-41. | |
|
|
Table 1 Structural parameters of mesoporous MCM-41 materials. |
Fig. 4 gives the effects of adsorbent amount and adsorption time on the DNA adsorption of metal ion-functionalized and bare MCM- 41. As seen from Fig. 4a,DNA adsorption efficiency onto the three modified MCM-41 samples was enhanced significantly compared with that onto bare MCM-41 with increasing adsorbent amount. These results prove the strong interaction between the metal ions and DNA. When the adsorbent amount was about 0.02 g,DNA adsorption was saturated. In this case,the degrees of DNA adsorption of Al3+-MCM-41,La3+-MCM-41,and Zn2+-MCM-41 were 90.39%,80.88%,and 70.40%,respectively. Therefore,the adsorption capacity of ion chelation on PO3--MCM-41 decreased according to the order Al3+-MCM-41 > La3+-MCM-41 > Zn2+- MCM-41. To determine the DNA adsorption equilibrium time of the functionalized MCM-41,adsorption curves were plotted. Fig. 4b illustrates the increase in the amount of adsorbed DNA molecules with increasing time. Among the samples,the adsorption efficiency of Al3+-MCM-41 was the highest. Maximum DNA adsorption was observed at approximately 20 min,which suggests that DNA adsorption reaches equilibrium in the three adsorbents. To ensure that DNA adsorption reaches equilibrium,an adsorption time of 30 min was selected for further experiments.

|
Download:
|
| Fig. 4.Effects of absorption conditions on DNA adsorption at room temperature: (a) adsorbent amount and (b) adsorption time. | |
How readily DNA elutes from modified MCM-41 surfaces is of equal importance to DNA adsorption in DNA purification applications. After saturated adsorption in different adsorbents,NaCl solution was used to elute the adsorbed DNA. Addition of an elution agent reduces the interaction between metal ions and DNA, thereby resulting in the release of DNA molecules. Fig. 5 shows the effects of desorption conditions on the recovery efficiency of DNA on Al3+-MCM-41,La3+-MCM-41,and Zn2+-MCM-41. For the pH desorption study (Fig. 5a),the 5 mL of NaCl solution with concentration of 3 mol L-1 was used to elute DNA. When the solution pH was elevated from 2 to 6,DNA desorption efficiency dramatically increased. At pH between 6 and 8,recovery efficiency varied slightly. As the pH was further increased,the degree of DNA desorption decreased sharply. Therefore,the pH of the eluent exerts an important effect on the desorption efficiency of DNA,and the maximum recovery efficiency was observed at pH × 6 in all samples studied. The effect of salt concentration on the DNA desorption behavior was further investigated to understand interactions between DNA and the particle surfaces (Fig. 5b). Results showed that recovery efficiency increased as the concentration of NaCl increased and that maximum efficiency was achieved when the NaCl concentration was 3-4 mol L-1. The effect of desorption time is illustrated in Fig. 5c. Test results indicate that the amount of DNA molecules eluted from the functionalized MCM-41 increases with prolonged desorption time and becomes constant after 15 min. Based on the results in Fig. 5,DNA recovery efficiency among the three samples decreases following the order Al3+-MCM-41 > La3+-MCM-41 > Zn2+-MCM-41.

|
Download:
|
| Fig. 5.Effects of eluent conditions on DNA recovery efficiency at room temperature: (a) pH, (b) elution concentration, and (c) desorption time. | |
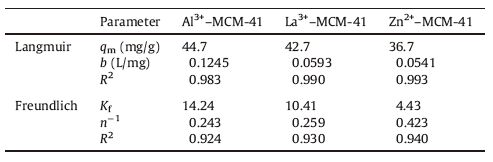
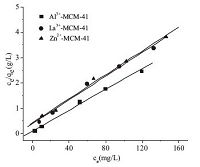
The Langmuir adsorption isotherms of Al3+-MCM-41,La3+- MCM-41,and Zn2+-MCM-41 are displayed in Fig. 6,and the corresponding calculated parameters are shown in Table 2. The adsorption data of the three absorbents fit the Langmuir equation well. As can be seen in Table 2,the maximum absorption capacities of Al3+-MCM-41,La3+-MCM-41,and Zn2+-MCM-41 at room temperature were 44.7,42.7,and 36.7 mg/g,respectively,thus showing the trend Al3+-MCM-41 > La3+-MCM-41 > Zn2+-MCM- 41. This trend is similar to the results shown in Fig. 4. The adsorption data were also fitted to the Freundlich isotherm model (Fig. 7). Analysis of the adsorption isotherms derived from the two models demonstrates that the Langmuir model fits the data better than the Freundlich model because the linear correlation coefficient obtained from the former is above 0.98. These results suggest that the adsorption mechanism is monolayer adsorption.
|
Download:
|
| Fig. 6.Langmuir adsorption isotherms of metal ion-functionalized MCM-41. | |
|
|
Table 2 Calculated Langmuir and Freundlich adsorption constants and correlation coefficients. |
The adsorption mechanism of DNA in the different adsorbents was further discussed. Generally,theMCM-41 surface is negatively charged due to the presence of silanol groups [27]; DNA molecules are also negatively charged at pH 7. Therefore,the pure mesoporous molecular sieve adsorbs very small amounts of DNA under neutral conditions. Though the surface area of bare MCM-41 is large (Table 1),very weak interactions exist between MCM-41 and DNA [28]. It was found that the surface area of metal ion functionalized MCM-41 is reduced significantly. Consequently,surface area has little impact on the adsorption of DNA. DNA could be adsorbed efficiently on metal ion-modified MCM-41 surfaces by coordination between the metal ion and the phosphate group of DNA [13]. The DNA adsorption experimental results show that the adsorption capacity of the adsorbents follows the order Al3+-MCM-41 > La3+- MCM-41 > Zn2+-MCM-41. According to the HSABtheory,hard acids prefer bindingwith hard bases andsoft acids prefer binding with soft bases. Al3+ is a hard acid because of its small ionic radius and high positive charge,and it can easily form a stable complex with a phosphate group of theDNAmolecule. Consequently,Al3+-MCM-41 has a greater tendency toadsorbDNAthanthe other adsorbents. La3+ is also a hard acidwith the same positive charge as Al3+,but its ionic radius is larger than that of Al3+. The interaction between La3+ and the phosphate group of DNA is weaker than that between Al3+ and the phosphate backbone because La3+ is a softer acid than Al3+. Thus, the adsorption efficiency of the La3+ chelation adsorbent is slightly lower than that of Al3+-MCM-41. The adsorption efficiency of Zn2+- MCM-41 is the lowest among the three adsorbents possibly because the binding force between Zn2+ and the phosphate group of DNA is the weakest,given that Zn2+ is a borderline acid. DNA desorption occurs as a result of the interaction of chloride ionswith metal ions. Based on HSAB theory,the acting force between Cl- and the metal ions Al3+,La3+,and Zn2+ weakens in turn. Therefore,desorption efficiency decreases in this order.

|
Download:
|
| Fig. 7.Freundlich adsorption isotherms of metal ion-functionalized MCM-41: (a) Al3+-MCM-41, (b) La3+-MCM-41, and (c) Zn2+-MCM-41. | |
In summary,three types of functionalized MCM-41 were successfully achieved through amino grafting of MCM-41 followed by phosphonate modification and metal ion chelation. The ability of the adsorbents to absorb and release DNA was then assessed. The DNA adsorption efficiency of the modified MCM-41 was enhanced significantly compared to that of pure MCM-41. Adsorption results showed that maximum adsorption increases following the trend Zn2+-MCM-41 < La3+-MCM-41 < Al3+-MCM- 41; these results indicate that Al3+ presents the strongest affinity toward DNA molecules. When the concentration of NaCl solution was 3 mol L-1 at pH 6,a large amount of DNA was eluted from the adsorbent at room temperature. The adsorption data suggested that monolayer adsorption could occur in the materials. This study offers a valid approach to DNA purification,which may lead to new directions in the enrichment of other biological samples containing phosphate groups.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21205070) and the Outstanding Youth Scientist Research Foundation of Shandong Province (No. 2007BS02003).
| [1] | P. Chomczynski, N. Sacchi, The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction:twenty-something years on, Nat. Protoc. 1(2006) 581-585. |
| [2] | H.K. Choi, J.H. Chang, I.H. Ko, et al., Electrostatic interaction effect for human DNA separation with functionalized mesoporous silicas, J. Solid State Chem. 184(2011) 805-810. |
| [3] | P.E. Vandeventer, J. Mejia, A. Nadim, M.S. Johal, A. Niemz, DNA Adsorption to and elution from silica surfaces:influence of amino acid buffers, J. Phys. Chem. B 117(2013) 10742-10749. |
| [4] | X.X. Huang, Z.M. Tao, J.C. Praskavich Jr., et al., Dendritic silica nanomaterials(KCC-1) with fibrous pore structure possess high DNA Adsorption capacity and effectively deliver genes in vitro, Langmuir 30(2014) 10886-10898. |
| [5] | H. Yang, K. Zheng, Z.M. Zhang, et al., Adsorption and protection of plasmid DNA on mesoporous silica nanoparticles modified with various amounts of organosilane, J. Colloid Interface Sci. 369(2012) 317-322. |
| [6] | N.M. Adams, H. Bordelon, K.K.A. Wang, et al., Comparison of three magnetic bead surface functionalities for RNA extraction and detection, ACS Appl. Mater. Interfaces 7(2015) 6062-6069. |
| [7] | W.W. Hu, Y.J. Chen, R.C. Ruaan, et al., The regulation of DNA adsorption and release through chitosan multilayers, Carbohydr. Polym. 99(2014) 394-402. |
| [8] | N. Sun, C.L. Deng, Y. Liu, et al., Optimization of influencing factors of nucleic acid adsorption onto silica-coated magnetic particles:application to viral nucleic acid extraction from serum, J. Chromatogr. A 1325(2014) 31-39. |
| [9] | R. Vathyanm, E. Wondimu, S. Das, et al., Improving the adsorption and release capacity of organic-functionalized mesoporous materials to drug molecules with temperature and synthetic methods, J. Phys. Chem. C 115(2011) 13135-13150. |
| [10] | M. Moritz, Solvent optimization for niacinamide adsorption on organo-functionalized SBA-15 mesoporous silica, Appl. Surf. Sci. 283(2013) 537-545. |
| [11] | J.L. Steinbacher, C.C. Landry, Adsorption and release of siRNA from porous silica, Langmuir 30(2014) 4396-4405. |
| [12] | P.E. Vandeventer, J.S. Lin, T.J. Zwang, et al., Multiphasic DNA adsorption to silica surfaces under varying buffer, pH, and ionic strength conditions, J. Phys. Chem. B 116(2012) 5661-5670. |
| [13] | S. Jiang, J.Q. Zhuang, C. Wang, J. Li, W.S. Yang, Highly efficient adsorption of DNA on Fe3+-iminodiacetic acid modified silica particles, Colloids Surf. A:Physicochem. Eng. Aspect. 409(2012) 143-148. |
| [14] | X. Li, J.X. Zhang, H.C. Gu, Study on the adsorption mechanism of DNA with mesoporous silica nanoparticles in aqueous solution, Langmuir 28(2012) 2827-2834. |
| [15] | T. Geng, N. Bao, O.Z. Gall, C. Lu, Modulating DNA adsorption on silica beads using an electrical switch, Chem. Commun.(2009) 800-802. |
| [16] | J.D. Zhang, Z.M. Shen, Z.J. Mei, S.P. Li, W.H. Wang, Removal of phosphate by Fecoordinated amino-functionalized 3D mesoporous silicates hybrid materials, J. Environ. Sci. 23(2011) 199-205. |
| [17] | R.H. Huang, J. Liu, L.S. Li, et al., Fe/MCM-41 as a promising heterogeneous catalyst for ozonation of p-chlorobenzoic acid in aqueous solution, Chin. Chem. Lett. 22(2011) 683-686. |
| [18] | J.H. Liu, D. Liang, B.B. Fan, R.F. Li, H. Chen, Enantioselective hydrogenation of acetophenone by(1S, 2S)-DPEN-Ru(Ⅱ)Cl2(PPh3)2 encapsulated in Al-MCM-41, Chin. Chem. Lett. 21(2010) 802-806. |
| [19] | L.H. Hu, H.J. Zhou, Y.H. Li, et al., Profiling of endogenous serum phosphorylated peptides by titanium(IV) immobilized mesoporous silica particles enrichment and MALDI-TOFMS detection, Anal. Chem. 81(2009) 94-104. |
| [20] | Z.Y. Ma, Y.P. Guan, H.Z. Liu, Superparamagnetic silica nanoparticles with immobilized metal affinity ligands for protein adsorption, J. Magn. Magn. Mater. 301(2006) 469-477. |
| [21] | Y.J. Jiang, Q.M. Gao, H.G. Yu, Y.R. Chen, F. Deng, Intensively competitive adsorption for heavy metal ions by PAMAM-SBA-15 and EDTA-PAMAM-SBA-15 inorganic-organic hybrid materials, Microporous Mesoporous Mater. 103(2007) 316-324. |
| [22] | M. Puanngam, F. Unob, Preparation and use of chemically modified MCM-41 and silica gel as selective adsorbents for Hg(Ⅱ) ions, J. Hazard. Mater. 154(2008) 578-587. |
| [23] | P. Selvam, S.K. Bhatia, C.G. Sonwane, Recent advances in processing and characterization of periodic mesoporous MCM-41 silicate molecular sieves, Ind. Eng. Chem. Res. 40(2001) 3237-3261. |
| [24] | X.G. Wang, S.K. Kyle, Lin, C.C. Jerry, S. Chan, Cheng, Direct synthesis and catalytic applications of ordered large pore aminopropyl-functionalized SBA-15 mesoporous materials, J. Phys. Chem. B 109(2005) 1763-1769. |
| [25] | L. Bois, A. Bonhommé, A. Ribes, et al., Functionalized silica for heavy metal ions adsorption, Colloids Surf. A:Physicochem. Eng. Asp. 221(2003) 221-230. |
| [26] | D.H. Everett, Manual of symbols and terminology for physicochemical quantities and units, appendix Ⅱ:definitions, terminology and symbols in colloid and surface chemistry, Pure Appl. Chem. 31(1972) 577-638. |
| [27] | X.X. He, K.M. Wang, W.H. Tan, et al., Bioconjugated nanoparticles for DNA protection from cleavage, J. Am. Chem. Soc. 125(2003) 7168-7169. |
| [28] | M. Fujiwara, F. Yamamoto, K. Okamoto, K. Shiokawa, R. Nomura, Adsorption of duplex DNA on mesoporous silicas:possibility of inclusion of DNA into their mesopores, Anal. Chem. 77(2005) 8138-8145. |
 2016, Vol.27
2016, Vol.27