b Shandong Academy of Pharmaceutical Sciences, Shandong Provincial Key Laboratory of Biomedical Polymer, Jinan 250101, China;
c Shandong Success Pharmaceutical Technology Co., Ltd., Jinan 250101, China
Polyurethanes (PUs) usually exhibit good mechanical properties and desirable blood compatibility,which are considered to be important characteristics for biomedical materials [1, 2, 3, 4]. For these advantages,PUs have been widely used in meniscal reconstruction [5],hemostatic sponge [6],vascular prosthesis [7] and artificial skin [8]. However,most commercially available PUs contain aromatic diphenylmethane diisocyanate (MDI) [9, 10, 11]. It is known that PUs based on MDI produce mutagenic and carcinogenic substances on degradation [12, 13]. In order to overcome this shortcoming,aliphatic diisocyanates are used to prepare PUs which only release non-toxic products on degradation. 1,4- Butanediisocyanate (BDI) is the optimal choice for its high chemical reactivity [14, 15],but BDI is prohibitively expensive. 1,6-Hexanediisocyanate (HDI) is much cheaper than BDI and has medium chemical reactivity,but the PUs exhibit mechanical properties unsatisfactory for medical applications when HDI is used as the chain extender [16, 17]. The development of biomedical PUs with good mechanical properties and low cost is therefore highly desirable [18, 19].
In this work,we wish to present new aliphatic PUs with low cost for biomedical applications. The prepolymer of poly-(ε-caprolactone- co-L-lactic acid)-poly(ethylene glycol)-poly-(ε-caprolactone- co-L-lactic acid) (PCLL-PEG-PCLL) was synthesized via bulk ring-opening polymerization with PEG600 as an initiator and L-lactide (L-LA),e-caprolactone (CL) as monomers. Then,the prepolymer was chain-extended with an isocyanate-terminated urethane triblock to obtain the PUs. The corresponding PU films containing long uniform-size hard segments had higher tensile strength than PU films extended with HDI and were more suitable for biomedical applications.
2. Experimental 2.1. Synthesis of prepolymer (PCLL-PEG-PCLL)21.5 g (0.15 mol) L-LA was mixed with 21.5 g (0.19 mol) CL in a 100 mL vacuum flask under nitrogen atmosphere,30.0 g (0.05 mol) PEG600 and 70 mg stannous octoate was added as initiator and catalyst,respectively. The mixture was polymerized under reduced pressure at 140 ℃ for 24 h. 1H NMR showed complete conversion.
1H NMR (400 MHz,CDCl3): δ 5.16 (q,CH3-CH-),4.13 (m,-COO- CH2-),3.65(m,CH2 ofPEG),2.34(m,-OOC-CH2-),1.44-1.57(br,CH3-, -COO-CH2-CH2-,-OOC-CH2-CH2-),1.41 (m,-OOC-(CH2)2-CH2-). GPC (THF): Mw-GPC = 1698,Mn-GPC = 1464,Mw-GPC/Mn-GPC = 1.16.
2.2. Synthesis of macrodiisocyanate chain extender1,6-Hexanediisocyanate-1,4-butanediol-1,6-hexanediisocyanate (HDI-BDO-HDI) was prepared by reaction of BDO with eightfold excess of HDI at 80 ℃ for 3 h without catalyst. After cooling to room temperature,the reaction mixture was washed three times by dry hexane to remove excess HDI. The product was dried at 45 ℃ under reduced pressure,yielding the HDI-BDO-HDI (88%) as a white powder.
1H NMR (400 MHz,DMSO-d6): δ 7.06 (t,2H,-NH-),3.93 (t,4H, -COO-CH2-),3.33 (t,4H,OCN-CH2-),2.95 (t,4H,-NH-CH2-),1.52- 1.56 (m,8H,-COO-CH2-CH2-,-NH-CH2-CH2-),1.39 (m,4H,OCN- CH2-CH2-),1.28 (m,4H,OCN-(CH2)2-CH2-). 13C NMR (400 MHz, DMSO-d6): δ 156.2 (-NH-CO-),121.5 (OCN-),63.1 (-COO-CH2-), 42.4 (OCN-CH2-),40.0 (-NH-CH2-),30.6 (OCN-CH2-CH2-),29.3 (-NH-CH2-CH2-),25.4-25.9 (OCN-(CH2)2-CH2-,OCN-(CH2)3-CH2-, -COO-CH2-CH2-). HR-MS (m/z): 449.24 [M+Na+].
2.3. Preparation of PUsThe chain extender of HDI-BDO-HDI was dissolved in dimethyl sulfoxide (DMSO) at 80 ℃ to get a 25 wt% solution,and the solution was added dropwise into the prepolymer under vigorous mechanical stirring (molar ratio of -NCO/-OH was 1.05). When the reaction mixture became viscous,a small amount of DMSO was added to keep the system homogeneous. After the addition was complete,the reaction mixture was stirred at 90 ℃ for about 3.5 h until the NCO peak (2270 cm-1) disappeared in the FT-IR spectrum, and subsequently diluted to a concentration of about 5 wt% solution. The polymer (PU-I) was precipitated in water and dried to constant mass at 35 ℃ under reduced pressure. The chemical structure of PU-I was shown in Scheme 1.
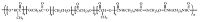
|
Download:
|
| Scheme 1.The chemical structure of PU-I. | |
For the purpose of comparison,PU-II was prepared with the same process except using HDI as chain extender.
IR (KBr,cm-1) for PU-I and PU-II: νmax 3319 (N-H),2933,2863 (C-H),1730,1687 (C=O),1195,1092 (C-O). GPC (THF) for PU-I: Mw-GPC = 147,000,Mn-GPC = 107,300,Mw-GPC/Mn-GPC = 1.37. GPC (THF) for PU-II: Mw-GPC = 82,600,Mn-GPC = 66,600,Mw-GPC/Mn-GPC = 1.24.
2.4. Polymer filmsThe PUs were dissolved in chloroform at room temperature under magnetic stirring for 3 h. Then the solution was poured into a polytetrafluoroethylene (PTFE) mold. The solvent was removed by natural volatilizing at room temperature for two days and subsequently transferred to a vacuum drying oven for one day so as to remove the last traces of solvent under reduced pressure.
3. Results and discussion 3.1. Polymer synthesisThe PCLL-PEG-PCLL copolymers were synthesized via bulk ringopening polymerization with stannous octoate as catalyst. The 1H NMR spectral peaks of the copolymers appearing at δ 5.16,δ4.13 and δ 3.65 ppm are attributed to the -CH of LA,ω-CH2 of CL and -CH2-CH2 of PEG units,respectively. The L-LA/CL molar ratio (1:1.23) could be determined by comparing the intensity of signals at δ 5.16 and δ 4.13 ppm,which matched the initial ratios (1:1.27) of the components used in the polymerization. The number molecular weight (Mn-NMR) of the copolymers was estimated by integrating the 1H NMR signals pertaining to each monomer according to the method established [20]. The Mn-NMR (1438 g/mol) was consistent with the theoretical molecular weight (Mtheo: 1457 g/mol) and the result of GPC (Mn-GPC: 1464 g/mol). All the results indicated the complete ring-opening reaction.
By chain extending the prepolymer (PCLL-PEG-PCLL) with the macrodiisocyanate (HDI-BDO-HDI),the PU-I with well-defined and uniform hard segments (HDI-BDO-HDI) was prepared. The uniformity of the hard segments is known to be extremely important for the mechanical properties. With macrodiisocyanate as chain extender,the PU-I with excellent mechanical properties and high molecular weight could be made.
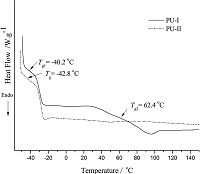
3.2. Thermal transitionFig. 1 shows the DSC thermograms of the PU films which were measured with a DSC2910 (Universal,USA) at a heating rate of 10 ℃/min from -50 ℃ to 150 ℃ under N2 (30 mL/min). Two glass transition temperatures (Tg1 = -40.2 ℃,Tg2 = 62.4 ℃) could be observed in the thermogram of PU-I extended with macrodiisocyanate, which corresponded to the soft ether segments and hard urethane segments,respectively. Because of the uniform longer hard segments contained in the PU,the hard phase was hardly miscible with the soft phase in the linear PU,which resulted in micro-phase separation and two clearly Tg being observed [21]. PU-II extended with HDI contains no long hard segments, as such it has only one Tg (-42.8 ℃) and no phase separation,as observed in the thermogram. In addition,no exothermic peaks in the thermograms of either PU indicated that PU-I and PU-II were amorphous.
3.3. Thermal stabilityFig. 2 shows the TG and DTG analysis of the PU films,which were heated at 20 ℃/min from 50 ℃ to 600 ℃ under air atmosphere (40 mL/min) with a TGA 2050 analyzer (Universal, USA) [22]. The PU-I and PU-II showed mass loss distributed in two steps. The first weight loss occurred at around 256-354 ℃ (PU-I: 68 wt%,Fig. 2a) and 250-364 ℃ (PU-II: 63 wt%,Fig. 2b) which was the decomposition of urethane and ester bonds in PUs. In the temperature region at around 354-456 ℃ (Fig. 2a) and 364-461 ℃ (Fig. 2b),the ether bonds decomposed almost completely,and the observed mass loss of 31 wt% and 36 wt% were consistent with the PEG content in PU-I (31.8 wt%) and PU-II (36.8 wt%),respectively. The TG curves of films having no obvious difference indicated that the PU-I and PU-II had similar thermal stability.
3.4. Mechanical propertiesThe mechanical properties of the PU films were measured with a single-column tensile test machine (Model HY939C,Hengyu Instruments,Ltd,Dongguan,China) at the cross-head speed of 50 mm/min according to the national standard GB/T1040-2006.The dumbbell-shaped specimens were cut from the films with a punching die of 12 mm width and 75 mm length. The neck width and length were 4.0 mm and 30 mm,respectively. The tensile properties of the PU films were presented in Fig. 3. The PU-I synthesized with HDI-BDO-HDI as chain extender had a tensile strength of 27.5 MPa with an elongation at break of 996%. The tensile strength of PU-II extended with HDI decreased significantly to 9.8 MPa,and the elongation at break increased slightly to 1090%. The much higher tensile strength was related with the phase separated morphology which was caused by the uniformity of the hard segments. Hard segments of uniform size packed better than non-uniform hard segments,and the more difficult to disrupt structure resulted in a higher tensile strength. The elongation at break could be affected by the soft segments,a slightly lower soft segment content in PU-I than PU-II led to a slightly higher elongation at break. The permanent deformation of the PU films was observed clearly in Fig. 3 (PU-I: 24.6%; PU-II: 13.3%),which were similar with the PU films synthesized with 1,4-butanediamine or BDO as chain extenders [8].
|
Download:
|
| Fig. 1.DSC thermograms of the PU films. | |

|
Download:
|
| Fig. 2.TG and DTG curves of (a) PU-I and (b) PU-II films. | |

|
Download:
|
| Fig. 3.Stress–strain behavior of PU films. | |
|
Download:
|
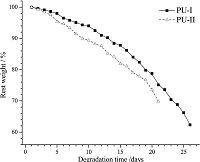
| Fig. 4.Degradation behaviors of PU films in physiological saline. | |
Films with a thickness of 0.2 × 0.02 mm were prepared by the solution casting method,as described above. 30 mm ± 30 mm samples were cut and then immersed in physiological saline for biodegradation tests. Biodegradation was allowed to proceed at a thermostated temperature (37 × 0.5 ℃). After predetermined periods of time,the samples were taken out,washed with distilled water,and vacuum dried at room temperature until constant weight. The test was carried out until the films lost mechanical properties and became fragments. The degradation behaviors of PU films are indicated in Fig. 4. The slower degradation rate of PU-I compared to PU-II maybe related to the lower hydrophilic segments (PEG) content and higher urethane segment content in PU-I. The increasing degradation rate of PU-I and PU-II should be due to the acid autocatalysis of the degradation products [23]. The pH of the solution containing PU-I and PU-II degradation products had decreased to 4.6 after 26 d,and 4.4 after 22 d. The PU-I film could maintain mechanical properties for more than three weeks,which could meet the requirement of biomedical applications.
4. ConclusionA biodegradable PU (PU-I) containing uniform longer hard segments was prepared. The macrodiisocyanate,prepolymer,and PUs were characterized by 1H NMR,13C NMR,HR-MS,FT-IR,GPC, TG,and DSC. Two clearly observed Tg in the DSC thermogram indicated a micro-phase separation in PU-I. The corresponding PU films showed excellent mechanical properties with a tensile strength of 27.5 MPa and an elongation at break of 996%,and also maintained mechanical properties in physiological saline at 37 ℃ for more than three weeks,which appeared to be more suitable for biomedical applications.
| [1] | C.V. Mythili, A.M. Retna, S. Gopalakrishnan, Synthesis, mechanical, thermal and chemical properties of polyurethanes based on cardanol, Bull. Mater. Sci. 27(2004) 235-241. |
| [2] | B. van Minnen, M.B.M. van Leeuwen, B. Stegenga, et al., Short-term in vitro and in vivo biocompatibility of a biodegradable polyurethane foam based on 1,4-butanediisocyanate, J. Mater. Sci. Mater. Med. 16(2005) 221-227. |
| [3] | N.J. Song, X. Jiang, J.H. Li, et al., The degradation and biocompatibility of waterborne biodegradable polyurethanes for tissue engineering, Chin. J. Polym. Sci. 31(2013) 1451-1462. |
| [4] | N.Y. He, C. Yang, Z.C. Liu, Z.H. Lu, Polyurethane molecular stamps for the in situ synthesis of DNA microarray, Chin. Chem. Lett. 13(2002) 883-886. |
| [5] | R.G.J.C. Heijkants, R.V. van Calck, J.H. de Groot, et al., Design, synthesis and properties of a degradable polyurethane scaffold for meniscus regeneration, J. Mater. Sci. Mater. Med. 15(2004) 423-427. |
| [6] | F.I. Broekema, W. van Oeveren, M.H.A. Selten, et al., In vivo hemostatic efficacy of polyurethane foam compared to collagen and gelatin, Clin. Oral Investig. 17(2013) 1273-1278. |
| [7] | W. He, Z.J. Hu, A.W. Xu, et al., The preparation and performance of a new polyurethane vascular prosthesis, Cell Biochem. Biophys. 66(2013) 855-866. |
| [8] | C.J. Spaans, J.H. de Groot, F.G. Dekens, A.J. Pennings, High molecular weight polyurethanes and a polyurethane urea based on 1,4-butanediisocyanate, Polym. Bull. 41(1998) 131-138. |
| [9] | A. Saralegi, A. Etxeberria, B. Fernández-d'Arlas, et al., Effect of H12 MDI isomer composition on mechanical and physico-chemical properties of polyurethanes based on amorphous and semicrystalline soft segments, Polym. Bull. 70(2013) 2193-2210. |
| [10] | V.K. Hridya, M. Jayabalan, Studies on in vitro biostability and blood compatibility of polyurethane potting compound based on aromatic polymeric MDI for extracorporeal devices, J. Mater. Sci. Mater. Med. 20(2009) 195-202. |
| [11] | R.C.S. Araújo, V.M.D. Pasa, Thermal study of polyurethane elastomers based on Biopitch-PEG-MDI system, J. Therm. Anal. Calorim. 67(2002) 313-319. |
| [12] | J.P. Santerre, K. Woodhouse, G. Laroche, R.S. Labow, Understanding the biodegradation of polyurethanes:from classical implants to tissue engineering materials, Biomaterials 26(2005) 7457-7470. |
| [13] | B.F. d'Arlas, L. Rueda, K. de la Caba, I. Mondragon, A. Eceiza, Microdomain composition and properties differences of biodegradable polyurethanes based on MDI and HDI, Polym. Eng. Sci. 48(2008) 519-529. |
| [14] | B. van Minnen, M.B.M. van Leeuwen, G. Kors, et al., In vivo resorption of a biodegradable polyurethane foam, based on 1,4-butanediisocyanate:a threeyear subcutaneous implantation study, J. Biomed. Mater. Res. A 85(2008) 972-982. |
| [15] | J.H. de Groot, C.J. Spaans, F.G. Dekens, A.J. Pennings, On the role of aminolysis and transesterification in the synthesis of e-caprolactone and L-lactide based polyurethanes, Polym. Bull. 41(1998) 299-306. |
| [16] | P.N. Shah, R.L. Manthe, S.T. Lopina, Y.H. Yun, Electrospinning of L-tyrosine polyurethanes for potential biomedical applications, Polymer 50(2009) 2281-2289. |
| [17] | P. Król, Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers, Prog. Mater. Sci. 52(2007) 915-1015. |
| [18] | V. Kanyanta, A. Ivankovic, Mechanical characterisation of polyurethane elastomer for biomedical applications, J. Mech. Behav. Biomed. Mater. 3(2010) 51-62. |
| [19] | C.J. Wu, A.K. Gaharwar, P.J. Schexnailder, G. Schmidt, Development of biomedical polymer-Silicate nanocomposites:a materials science perspective, Materials 3(2010) 2986-3005. |
| [20] | M.D. Lang, J.Z. Bei, S.G. Wang, Synthesis and characterization of polycaprolactone/poly(ethylene oxide)/polylactide tri-component copolymers, J. Biomater. Sci. Polym. Ed. 10(1999) 501-512. |
| [21] | P. Król, B. Pilch-Pitera, Phase structure and thermal stability of crosslinked polyurethane elastomers based on well-defined prepolymers, J. Appl. Polym. Sci. 104(2007) 1464-1474. |
| [22] | Z.S. Hou, C.Y. Kan, Preparation and properties of thermoexpandable polymeric microspheres, Chin. Chem. Lett. 25(2014) 1279-1281. |
| [23] | S.M. Li, Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids, J. Biomed. Mater. Res. 48(1999) 342-353. |
 2016, Vol.27
2016, Vol.27 


