1,2,3-Triazoles are an important class of heterocyclic compounds, which were widely applied in various fields including synthetic organic chemistry [1],biological science [2],medicinal chemistry [3] and material science [4]. In particular,they are found in clinical and commercial drugs such as IDO (indoleamine 2,3- dioxygenase) inhibitors [5],antibiotics [6],HDIs (histone deacetylase inhibitors) [7] and antiviral drugs (Fig. 1) [8].

|
Download:
|
| Fig. 1.Some 1,2,3-triazoles possessing pharmaceutical activities. | |
Because of their utility,several syntheses of 1,2,3-triazoles have been reported. The first method to form 1,2,3-triazole was the Huisgen dipolar cycloaddition,giving 1,4- and 1,5-disubstituted regioisomers [9]. In 2002,Sharpless [10] group found a coppercatalyzed 1,3-dipolar cycloaddition reaction (CuAAC) between alkynes and azides,allowing the regioselective formation of the 1,4-disubstituted 1,2,3-triazoles. From then on these compounds come to the limelight,bringing researchers to explore more effective methods using different approaches [11]. For example, the Fokin [12] group used triazole ligands TBAB to stabilize Cu(I), which can vigorously catalyze the Huisgen cycloaddition reaction to synthesize the 1,4-substituted 1,2,3-triazoles at room temperature. The Orgueira [13] group reported that the active nano-copper can also catalyze the Huisgen cycloaddition reaction. And this reaction can be carried out in various solvents such as THF,MeOH, MeCN,DMSO,DMF and so on [14]. Recently,the Ramachary [15] group reported an organocatalytic enolate-mediated synthesis of 1,2,3-triazoles from aldehydes and aryl azides,which constitutes an alternative methodology. Moreover,the synthesis of other derivatives such as 1-mono [16],4-mono [17],1,5-di [18],and 1,4,5-trisubsituted 1,2,3-triazoles [19] have also been reported. At the same time,some facile one-pot syntheses were demonstrated using aryldiazonium silica sulfates [20],aryl boronic acids [21], aryl halides [22] and aromatic amines [23] as the starting materials.
Based on our previous report on a one-pot synthesis of aryl azides from nitrobenzes [24],we would like to describe a convenient,efficient and economical one-pot method for the preparation of 1,4-disubstituted 1,2,3-triazoles 3 (Scheme 1),using aromatic nitrocompounds 1 and terminal alkynes 2 as the starting materials by a four-step one-pot sequence.

|
Download:
|
| Scheme 1.Some 1,2,3-triazoles possessing pharmaceutical activities. | |
1H NMR and 13C NMR spectra were recorded using a MercuryPlus 300 (300 MHz) or a Bruker ACF400 spectrometer (400 MHz) in CDCl3 using TMS as an internal standard. Chemical shifts for protons are reported in ppm downfield from the tetramethylsilane signal and are referenced to residual 1H in the NMRsolvent (CHCl3:TMS). Chemical shifts for carbons are reported in ppm downfield from the tetramethylsilane signal and are referenced to the carbon resonance of the solvent (CDCl3,δ 77.00). All reactions were monitored by TLC analysis using Huanghai GF254 silica gel coated plates. Column chromatography was carried out using 300-400 mesh silica gel at medium pressure. Infrared spectra were taken on a Bruker Vertex Series FTIR (KBr) and are reported in reciprocal centimeters (cm-1). Melting points were obtained using a Bu¨ chi melting point apparatus and are uncorrected. HRMS spectra were recorded on Waters Micromass Premier Q-TOF spectrometer.
General procedure: A mixture of nitrobenzenes 1 (1 mmol) and Zn (215 mg,3.3 mmol) in solvent of HOAc-H2O (2.5 mL,v/v=2:3) in a flask was stirred at room temperature until the starting nitrobenzenes were consumed completely (monitored by TLC analysis). NaNO2 (1.1 mmol) saturated solution was added dropwise at 0-5 ℃ in an ice-water bath followed by adding a 1.5 mmol of NaN3 saturated solution. Then the ice-water bath was removed and the reaction proceeded at room temperature. After 2 h,terminal alkynes 2 (1.2 mmol),CuI (0.05 mmol) and DMSO (1.5 mL) were added to the above system at room temperature. After 5 h,the mixture was treated with H2O (15 mL) and extracted with EtOAc (3 × 15 mL) and the combined organic layer was washed with brine (3 × 5 mL),dried over Na2SO4 and concentrated under reduced pressure to afford a crude product. Purification by column chromatography on silica gel afforded the desired 1,4- disubstitued 1,2,3-triazol 3.
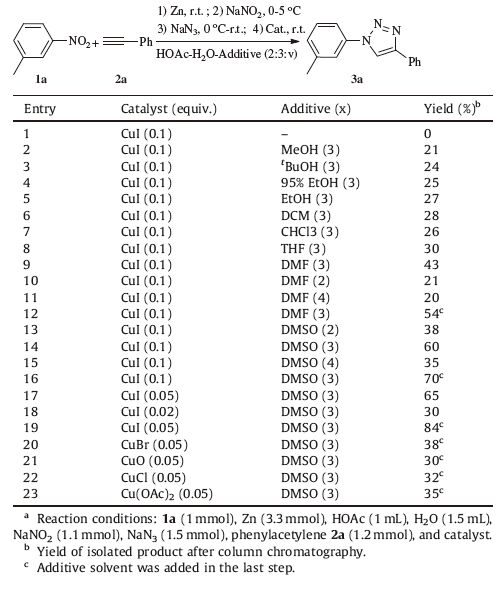
3. Results and discussionAn initial investigation of the reaction conditions was conducted using 1-methyl-3-nitrobenzene 1a and phenylacetylene 2a as the starting materials (Table 1). The first three steps of the reaction (including reduction,diazotization,and azidization) can go smoothly in the solvent of a HOAc-H2O-additive mixture (2:3:x,v/v),but no target molecule of 4-phenyl-1-(m-tolyl)-1H- 1,2,3-triazole 3a was detected when no additive solvent was present (Table 1,entry 1). When additive solvents such as MeOH, CHCl3,THF,DCM and EtOH were used,the product can be obtained although the yields were low (Table 1,entries 2-8). And higher yields of 43% and 60% were obtained respectively when 3 portions (in volume) of DMF or DMSO were used as the additive solvent (Table 1,entries 9,14),while only lower yields can be obtained when more or less amount of additive solvent was used (Table 1, entries 9-15). The effect of the amount of catalyst was also tested (Table 1,entries 17,18),and an improved yield of 3a was obtained when 0.05 equiv. of CuI was added using DMSO as an additive (Table 1,entry 17). However,only 30% yield was obtained when 0.02 equiv. of CuI was added (Table 1,entry 18). To our delight,the yields can be increased if the additive solvent was added in the last step (Table 1,entry 9 vs. 12,14 vs. 16). Additionally,we examined some other copper catalysts such as CuBr,CuO,CuCl and Cu(OAc)2, finding they were less efficient for this system (Table 1,entries 20- 23). As a result,the optimal conditions appeared 0.05 equiv. of CuI using HOAc-H2O-DMSO (2:3:3,v/v) as the solvent,with DMSO being added in the last step (Table 1,entry 19).
|
|
Table 1 Optimization of the reaction conditions.a |
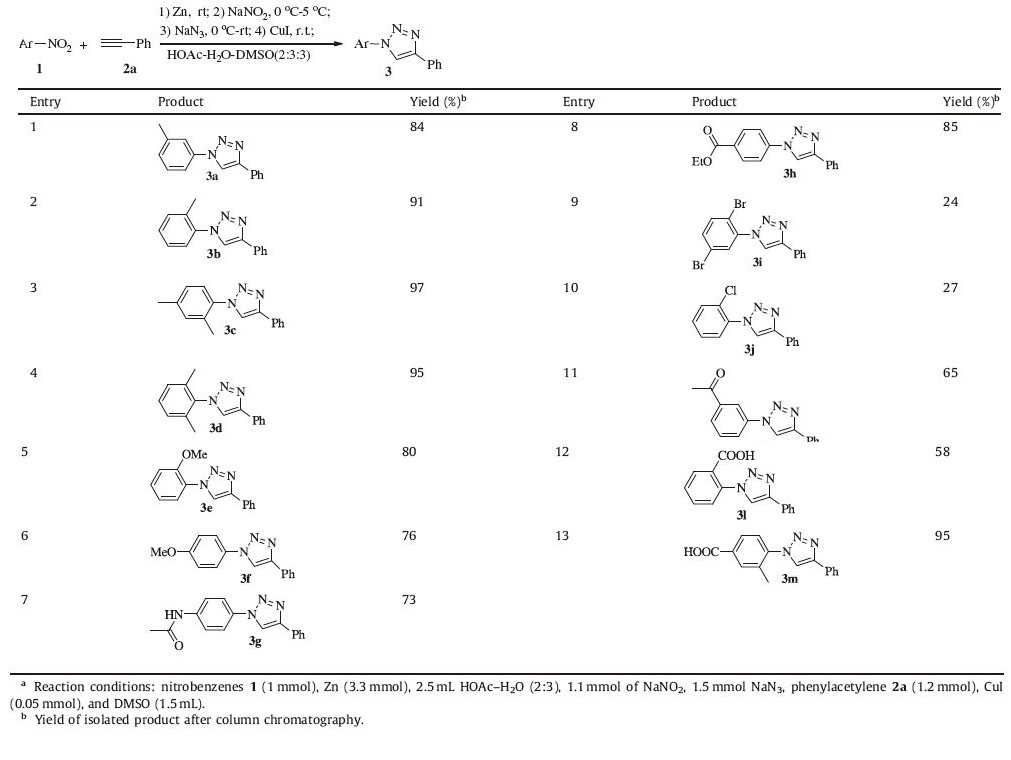
With the optimized reaction conditions in hand,a series of nitrobenzenes 1 and phenylacetylene 2a were then subjected to the reaction,affording the corresponding 1,4-disubstituted 1,2,3- triazoles 3. The results summarized in Table 2 showed that nitrobenzenes carrying either an electron donating substituent such as methyl,methoxy and amido group (Table 2,entries 1-7) or an electron withdrawing group including halogen,alkoxycarbonyl, carbonyl and carboxyl (Table 2,entries 8-13) could proceed successfully. Substrates bearing an electron-donating group at the para- or ortho-position of the nitrobenzenes could all give the corresponding 1,2,3-triazoles in good to excellent yields (Table 2, entries 5,6). Additionally,the functional groups in the nitrobenzenes such as carbonyl,alkoxycarbonyl,and carboxyl were tolerated under the conditions (Table 2,entries 11-13). However, the yields were somehow lower when the substituents contain Cl or Br (Table 2,entries 9,10).
|
|
Table 2 Synthesis of 1,4-disubstituted 1,2,3-triazoles 3 by expanding aromatic nitrocompounds.a |
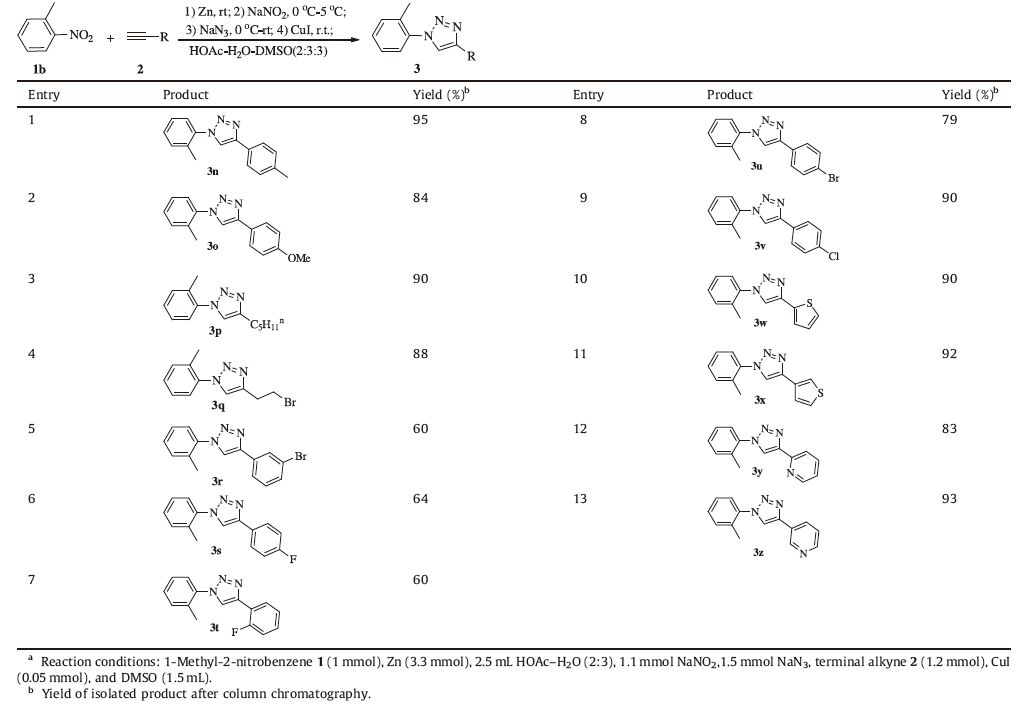
We also examined the scope of terminal alkynes,as summarized in Table 3. 1-Methyl-2-nitrobenzene 1b and terminal alkynes 2 were allowed to react in one pot under the optimal conditions to give the desired 1,4-disubstitued 1,2,3-triazols. To our delight, various substituted terminal alkynes,regardless of the substitution patterns (with electron-withdrawing or donating groups),affording the desired products in moderate to high yields (Table 3,entries 1-13). It is noteworthy that heterocycles such as ethynylpyridine and ethynyl thiophene were also tolerated as substrates to afford the desired products in high yields (Table 3,entries 10-13). Additionally,the reaction of terminal alkynes carrying electrondonating and electron-withdrawing group (Cl,Br) at the paraposition could also furnish the 1,2,3-triazoles in high yields (Table 3,entries 8,9). Alkyl terminal alkynes can also be employed in this system,providing the desired products in excellent yields (Table 3,entries 3,4).
|
|
Table 3 Synthesis of 1,4-disubstituted 1,2,3-triazoles 3 by expanding terminal alkynes.a |
In summary,we have developed a highly efficient protocol for the synthesis of 1,4-disubstitued 1,2,3-triazoles using nitrobenzenes and terminal alkynes as the starting materials,in which a four-step one-pot sequence was involved under mild conditions. It is an economical and facile method for the preparation of 1,2,3- triazole derivatives,which are widely applied in many fields.
AcknowledgmentsThe authors would like to thank the National Natural Science Foundation of China (Nos. 21262020 and 51464021) and the Science and Technology Planning Project of Yunnan Province (No. KKSY201207140) for the financial support.
| [1] | (a) D.J. Lee, E.J. Yoo, Efficient synthesis of C-N-coupled heterobiaryls by sequential N-H functionalization reactions, Org. Lett. 17(2015) 1830-1833;(b) T. Ryu, Y. Baek, P.H.J. Lee, Synthesis of pyrazines from rhodium-catalyzed reaction of 2H-azirines with N-sulfonyl 1,2,3-triazoles, J. Org. Chem. 80(2015) 2376-2383;(c) S. Shi, C. Kuang, Palladium-catalyzed ortho-alkoxylation of 2-aryl-1,2,3-triazoles, J. Org. Chem. 79(2014) 6105-6112. |
| [2] | (a) C. Testa, M. Scrima, M. Grimaldi, et al., 1,4-Disubstituted-[1,2,3] triazolylcontaining analogues of MT-Ⅱ:design, synthesis, conformational analysis, and biological activity, J. Med. Chem. 57(2014) 9424-9434;(b) P. Thirumurugan, D. Matosiuk, K. Jozwiak, Click chemistry for drug development and diverse chemical-biology applications, Chem. Rev. 113(2013) 4905-4979. |
| [3] | (a) S.G. Agalave, S.R. Maujan, V.S. Pore, Click chemistry:1,2,3-triazoles as pharmacophores, Chem. Asian J. 6(2011) 2696-2718;(b) R.W. Robey, A.R. Chakraborty, A. Basseville, et al., Histone deacetylase inhibitors:emerging mechanisms of resistance, Mol. Pharm. 8(2011) 2021-2031;(c) C. Sheng, W. Zhang, New lead structures in antifungal drug discovery, Curr. Med. Chem. 18(2011) 733-766. |
| [4] | (a) T. Muller, S. Brase, Click chemistry finds its way into covalent porous organic materials, Angew. Chem. Int. Ed. 50(2011) 11844-11845;(b) Y.H. Lau, P.J. Rutledge, M. Watkinson, et al., Chemical sensors that incorporate click-derived triazoles, Chem. Soc. Rev. 40(2011) 2848-2866;(c) C. Chu, R. Liu, Application of click chemistry on preparation of separation materials for liquid chromatography, Chem. Soc. Rev. 40(2011) 2177-2188. |
| [5] | U.F. Rö hrig, S.R. Majjigapu, A. Grosdidier, et al., Rational design of 4-aryl-1,2,3-triazoles for indoleamine 2,3-dioxygenase 1 inhibition, J. Med. Chem. 55(2012) 5270-5290. |
| [6] | M.A. Totir, P.S. Padayatti, M.S. Helfand, et al., Effect of the inhibitor-resistant M69V substitution on the structures and populations of trans-enamine(-lactamase intermediates), Biochemistry 45(2006) 11895-11904. |
| [7] | J. Bian, L. Zhang, Y. Han, C. Wang, L. Zhang, Histone deacetylase inhibitors:potent anti-leukemic agents, Curr. Med. Chem. 22(2015) 2065-2074. |
| [8] | K.E. Akri, K. Bougrin, J. Balzarini, A. Faraj, R. Benhida, Efficient synthesis and in vitro cytostatic activity of 4-substituted triazolyl-nucleosides, Bioorg. Med. Chem. Lett. 17(2007) 6656-6659. |
| [9] | R. Huisgen, Centenary lecture. 1,3-Dipolar cycloadditions, Proc. Chem. Soc.(1961) 357-369. |
| [10] | V.V. Rostovtsev, L.G. Green, V.V. Fokin, K.B. Sharpless, A stepwise huisgen cycloaddition process:copper(I)-catalyzed regioselective ligation of azides and terminal alkynes, Angew. Chem. Int. Ed. 41(2002) 2596-2599. |
| [11] | (a) X.J. Quan, Z.H. Ren, Y.Y. Wang, Z.H. Guan, p-Toluenesulfonic acid mediated 1,3-dipolar cycloaddition of nitroolefins with NaN3 for synthesis of 4-aryl-NH-1,2,3-triazoles, Org. Lett. 16(2014) 5728-5731;(b) A. Bolje, D. Urankar, J. Kosmrlj, Synthesis and NMR analysis of 1,4-disubstituted 1,2,3-triazoles tethered to pyridine, pyrimidine, and pyrazine rings, Eur. J. Org. Chem. 36(2014) 8167-8181. |
| [12] | T.R. Chan, R. Hilgraf, K.B. Sharpless, V.V. Fokin, Polytriazoles as copper(I)-stabilizing ligands in catalysis, Org. Lett. 6(2004) 2853-2855. |
| [13] | H.A. Orgueira, D. Fokas, Y. Isome, et al., Regioselective synthesis of[1,2,3]-triazoles catalyzed by Cu(I) generated in situ from Cu(0) nanosize activated powder and amine hydrochloride salts, Tetrahedron Lett. 46(2005) 2911-2914. |
| [14] | (a) D. Wang, L. Etienne, M. Echeverria, S. Moya, D. Astruc, A highly active and magnetically recoverable tris(triazolyl)-CuI catalyst for alkyne-azide cycloaddition reactions, Chem. Eur. J. 20(2014) 4047-4054;(b) A. Pathigoolla, R.P. Pola, K.M. Sureshan, A versatile solvent-free azide-alkyne click reaction catalyzed by in situ generated copper nanoparticles, Appl. Catal. A Gen. 453(2013) 151-158. |
| [15] | D.B. Ramachary, A.B. Shashank, S. Karthik, An organocatalytic azide-aldehyde[3+2] cycloaddition:high-yielding regioselective synthesis of 1,4-disubstituted 1,2,3-triazoles, Angew. Chem. Int. Ed. 53(2014) 10420-10424. |
| [16] | (a) Y. Jiang, C. Kuang, Q. Yang, The use of calcium carbide in the synthesis of 1-monosubstituted aryl 1,2,3-triazole via click chemistry, Synlett 19(2009) 3163-3166;(b) L. Tao, L.L. Zhang, S.J. Shen, X.P. Hang, Microwave-promoted rapid synthesis of 1-aryl-1,2,3-triazoles, Chin. Chem. Lett. 12(2001) 763-764. |
| [17] | (a) Y. He, E. Sun, Y. Zhao, L. Hai, Y. Wu, The one-pot synthesis of 4-aryl-1H-1,2,3-triazoles without azides and metal catalization, Tetrahedron Lett. 55(2014) 111-115;(b) X. Wang, C. Kuang, Q. Yang, Copper-catalyzed synthesis of 4-aryl-1H-1,2,3-triazoles from 1,1-dibromoalkenes and sodium azide, Eur. J. Org. Chem. 2(2012) 424-428. |
| [18] | (a) S.W. Kwok, J.R. Fotsing, R.J. Fraser, V.O. Rodionov, V.V. Fokin, Transitionmetal-free catalytic synthesis of 1,5-diaryl-1,2,3-triazoles, Org. Lett. 12(2010) 4217-4219;(b) X.Z. Cheng, W. Liu, Z.D. Huang, Sodium hydride-mediated synthesis of 1,5-diaryl-1,2,3-triazoles from anti-3-aryl-2,3-dibromopropanoic acids and organic azides, Chin. Chem. Lett. 24(2013) 764-766. |
| [19] | (a) G.C. Davir, S.I. Itzel, G.G. Carlos, et al., A novel and facile synthesis of 1,4,5-trisubstituted 1,2,3-triazoles from benzylic alcohols through a one-pot, threecomponent system, Tetrahedron Lett. 56(2015) 514-516;(b) Z.G. Luo, Y. Zhao, F. Xu, et al., Synthesis and thermal properties of novel calix[4] arene derivatives containing 1,2,3-triazole moiety via K2CO3-catalyzed 1,3-dipolar cycloaddition reaction, Chin. Chem. Lett. 25(2014) 1346-1348. |
| [20] | A. Zarei, One-pot, efficient, and regioselective syntheses of 1,4-disubstituted 1,2,3-triazoles using aryldiazonium silica sulfates in water, Tetrahedron Lett. 53(2012) 5176-5179. |
| [21] | B.S.P.A. Kumar, K.H.V. Reddy, K. Karnakar, et al., Copper on chitosan:an efficient and easily recoverable heterogeneous catalyst for one pot synthesis of 1,2,3-triazoles from aryl boronic acids in water at room temperature, Tetrahedron Lett. 56(2015) 1968-1972. |
| [22] | Y. Chen, Z.J. Zhuo, D.M. Cui, C. Zhang, Copper catalyzed synthesis of 1-aryl-1,2,3-triazoles from aryl iodides, alkynes, and sodium azide, J. Organomet. Chem. 749(2014) 215-218. |
| [23] | S. Guo, M.H. Lim, H.V. Huynh, Copper(I) heteroleptic bis(NHC) and mixed NHC/phosphine complexes:syntheses and catalytic activities in the one-pot sequential CuAAC reaction of aromatic amines, Organometallics 32(2013) 7225-7233. |
| [24] | F. Zhao,Z. Chen, P. Lei, L. Kong, Y. Jiang, Facile one-pot synthesis ofaryl azides from nitrobenzenes, Tetrahedron Lett. 56(2015) 2197-2199. |
 2016, Vol.27
2016, Vol.27