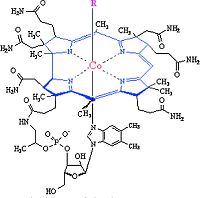
Vitamin B12,also called cobalamin (Cbl),Cbl has been called nature’s most beautiful cofactor and was identified as the antipernicious anemia factor from liver in 1948. Cbl shows biological activity in a very small amount. Cbl acts as the cofactor for two enzymes,i.e.,methylmalonyl-CoA mutase and methionine synthase,in humans. Both enzymes are important for health. The B12 content in food is very low. Its greatest abundance is in meat,fish,and milk products; it is generally absent from fruits and vegetables,but nor,an edible green and purple seaweed,is a nondairy product that contains a significant amount of B12 Cbl found in food is originally from these bacteria,in which the complex molecule is synthesized using at least 25 genes in the Cob operon. Cbl acts as the cofactor for two enzymes present in humans [1] (Fig. 1). The properties and reactivity of vitamin B12 derivatives have been extensively investigated ever since it was shown to possess a cobalt-carbon bond in the biological cofactors adenosyl and methylcobalamin [2, 3, 4, 5, 6]. These versatile organometallic complexes are used in a variety of enzymes to catalyze radical rearrangements and methyltransfers. Moreover,vitamin B12 has found use in organic chemistry for carbon-carbon bond formations [7, 8, 9, 10, 11, 12].
|
Download:
|
| Fig. 1.The structure of vitamin B12. | |
Furanones are the five-member heterocyclic compounds possessing lactone ring in their structures. These heterocycles are the core structures of many bioactive natural products as well as synthetic drugs such as rubrolide,sarcophine,benfurodilhemisuccinate,etc. [13, 14]. 5H-Furan-2-onederivatives exhibit many pharmacological and biological activities including antifungal,antibacterial,anti-oxidants,anti-inflammatory,anti-microbial and anti-cancer agents [15, 16, 17, 18, 19]. Due to this wide range of abundance and applicability,various approaches toward substituted butenolides have been developed,which involve the use of organolithium [20],boronic acids [21, 22],transition-metal catalysts such as Pd(OAc)2 [23],Ru [24],Cu(II) [25],AuCl [26],and secondary amines [27]. However,many of these methods involve the use of expensive catalysts and hazardous reagents in stoichiometric amounts. A new route to the synthesis of furan skeletons was developed by Murthy et al. via the multi-component reaction of aromatic amines,aldehydes and acetylenic esters,which lead to the preparation of 3,4,5-substituted furan-2(5H)-one derivatives using β-cyclodextrin as a catalyst in water [28]. Recently,Nagarapu et al. reported that SnCl2 can efficiently catalyze this reaction [29]. The presence of pyrrol-2-ones (5-lactams or glactams) in pharmaceuticals and natural products has continued to stimulate a great deal of interest in the development of new methodologies for their synthesis [30, 31]. There are several bioactive natural molecules with a pyrrol-2-one moiety,such as holomycin and thiolutin [32],thiomarinol A4 [33],oteromycin [34],pyrrocidine A [35],quinolactacin C [36],and ypaoamide [37]. On the other hand,dihydropyrrol-2-ones have been successfully used as peptidomimetic [38],HIV integrase [39],herbicidals [40],DNA polymerase inhibitors [41],caspase-3 inhibitors [42] cytotoxic and antitumor agents [43],antibiotics [44],and also inhibitors of the annexin A2-S100A10 protein interaction [45]. Recently,a few methods have been reported for the synthesis of highly substituted dihydropyrrol-2-ones using one-pot,fourcomponent reactions in the presence of catalyst,such as AcOH,I2,benzoic acid,TiO2 nanopowder or Cu(OAc)2 _H2O [46, 47, 48, 49, 50, 51].
However,some of these methods have drawbacks,such as high temperature and utilize a chlorinated solvent. Therefore,the development of a milder and more efficient route for one-pot synthesis of these important heterocycles is still in demand. Thus,in continue of our research on multi-component synthesis [52-=],we herein report a green synthesis of 3,4,5-trisubstituted furan- 2(5H)-ones and N-aryl-3-aminodihydropyrrol-2-one-4-carboxylates using catalytic amount of vitamin B12 as catalyst at ambient temperature in EtOH (Schemes 1 and 2).

|
Download:
|
| Scheme 1.Synthesis of 3,4,5-trisubstituted furan-2(5H)-ones in the presence of vitamin B12 as catalyst in EtOH at ambient temperature. | |
|
Download:
|
| Scheme 2.Synthesis of N-aryl-3-aminodihydropyrrol-2-one-4-carboxylates in the presence of vitamin B12 as catalyst in EtOH at ambient temperature. | |
Chemicals were purchased from Merck (Darmastadt,Germany),Acros (Geel,Belgium) and Fluka (Buchs,Switzerland),and used without further purification. The vitamin B12 was purchased from the Sigma-Aldrich company. Melting points were taken on an Electrothermal 9100 apparatus. IR spectra were obtained on a JASCO FT/IR-460 plus spectrometer. The 1H NMR and 13C NMR spectra were recorded on a Bruker DRX-400 Avanve instrument with CDCl3 as solvent and using TMS as internal reference at 400 MHz and 100 MHz,respectively.
2.2. General procedure for the synthesis of 3,4,5-trisubstituted furan-2(5H)-ones A mixture of amine 1 (1 mmol),dialkylacetylenedicarboxylate 2 (1 mmol),aromatic aldehyde 3 (1 mmol) and vitamin B12 (0.001 g) in EtOH (2 mL) was stirred at ambient temperature for appropriate time (Scheme 1). After completion of the reaction (monitored by TLC),the water was added to produce solid precipitate,and the precipitate was filtered off and washed with ethanol (3 × 2 mL) to give the pure product 4. The structures of the synthesized compounds were characterized by their IR,1H NMR and 13C NMR spectra and were found to be identical with data described in the literature.
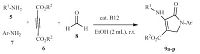
2.3. General procedure for the synthesis of N-aryl-3-aminodihydropyrrol-2-one-4-carboxylates A mixture of amine 5 (1 mmol) and dialkylacetylenedicarboxylate 6 (1 mmol) in EtOH (2 mL) was stirred for 25 min. Next,amine 7 (1 mmol),formaldehyde 8 (37% solution,1.5 mmol) and vitamin B12 (0.001 g) were added in successively. The reaction mixture was allowed to stir at ambient temperature for appropriate time. After completion of the reaction (monitored by TLC),the water was added to produce solid precipitate,and the precipitate was filtered off and washed with ethanol (3 × 2 mL) to give the pure product 9 (Scheme 2). The structures of the synthesized compounds were characterized by their IR,1H NMR and 13C NMR spectra and were found to be identical with data described in the literature [47, 48].
Methyl 2,5-dihydro-5-oxo-2-phenyl-4-(phenylamino)furan-3- carboxylate (4a): White solid; IR (KBr,cm-1): 3260,3208,1702,1661; 1H NMR (400 MHz,CDCl3): δ 3.77 (s,3 H,OCH3),5.76 (s,1H,benzylic),7.13 (t,1H,J = 7.3 Hz),7.24-7.31 (m,7H),7.52 (d,2H,J = 8 Hz),8.90 (br,NH,1H); 13C NMR (100 MHz,CDCl3): δ 165.3 and 162.7 (CO),156.3,136.1,134.9,129.0,128.7,128.6,127.4,125.9,122.3,112.8 (C of aromatic),61.6 (C of methoxy),52.1 (C of benzylic).
Methyl 4-(p-tolylamino)-2,5-dihydro-5-oxo-2-phenylfuran-3- carboxylate (4b): White solid; IR (KBr,cm-1): 3228,2950,1706,1677,1513; 1H NMR (400 MHz,CDCl3): δ 2.27 (s,3H,CH3),3.76 (s,3H,OCH3),5.72 (s,1H,benzylic),7.09 (d,2H,J = 8 Hz),7.22-7.270 (m,5H,aromatic),7.34 (d,2H,J = 8.4 Hz),8.86 (br,1H,NH); 13C NMR(100 MHz,CDCl3): δ 165.3 and 162.8 (CO),156.4,135.8,135.0,133.5,129.6,128.6,128.5,127.5,122.4,112.6 (C of aromatic),61.3 (C of methoxy),52.0 (C of benzylic),20.9 (C of methyl).
Ethyl 2-(4-cyanophenyl)-2,5-dihydro-5-oxo-4-(phenylamino)- furan-3-carboxylate (4e): White solid; IR (KBr,cm-1): 3293 (NH),2977,2225 (CN),1731,1684,1666,1500; 1H NMR (400 MHz,CDCl3): δ 1.23 (t,3H,J = 7.2 Hz,CH3),4.24 (q,2H,J = 7.2 Hz,CH2),5.82 (s,1H,benzylic),7.17 (t,1H,J = 7.2 Hz),7.32-7.47 (m,6H,aromatic),7.59 (d,2H,J = 8 Hz),9.03 (br,1H,NH); 13C NMR (100 MHz,CDCl3): δ 164.6 and 162.5 (CO),156.89,140.8,135.7,132.5,129.2,128.3,126.3,122.1,118.1,112.6 (C of aromatic),112.2 (C of CN),61.6 (C of methoxy),60.8 (C of benzylic),14.0 (C of ethoxy).
Methyl 2,5-dihydro-5-oxo-1-phenyl-4-(phenylamino)-1H-pyrrole- 3-carboxylate (9a): White solid,IR (KBr,cm-1): 3310 (NH),1705,1684,1645; 1H NMR (400 MHz,CDCl3): δ 3.76 (s,3H,OCH3),4.57 (s,2H,CH2),7.16-7.23 (m,4H,ArH),7.34 (t,2H,J = 8.0 Hz,ArH),7.42 (t,2H,J = 8.0 Hz,ArH),7.81 (d,2H,J = 8.0 Hz,ArH),8.05 (br s,1H,NH).
Ethyl 4-(p-tolylamino)-2,5-dihydro-5-oxo-1-p-tolyl-1H-pyrrole- 3-carboxylate (9d): Yellow solid,IR (KBr,cm-1): 3310 (NH),1707,1682,1649; 1H NMR (400 MHz,CDCl3): δ 1.25 (t,3H,J = 7.2 Hz,OCH2CH3),2.36 (s,3H,CH3),2.37 (s,3H,CH3),4.23 (t,2H,J = 7.2 Hz,OCH2CH3),4.52 (s,2H,CH2),7.06 (d,2H,J = 8.4 Hz,ArH),7.14 (d,2H,J = 8.0 Hz,ArH),7.21 (d,2H,J = 8.4 Hz,ArH),7.69 (d,2H,J = 8.8 Hz,ArH),8.01 (br s,1H,NH); 13C NMR (100 MHz,CDCl3): d 164.7 and163.7 (CO),143.1,136.3,136.2,134.6,134.2,129.6,128.9,122.9,119.1,102.4 (C of aromatic),48.3 (CH2-N),60.2 (C of methoxy),21.0 (Cof methyl),20.9 (C of methyl),14.2 (C of ethoxy).
Methyl 4-(benzylamino)-1-(4-fluorophenyl)-2,5-dihydro-5- oxo-1H-pyrrole-3-carboxylate (9k): White solid,IR (KBr,cm-1): 3310 (NH),1704,1682,1646; 1H NMR (400 MHz,CDCl3): δ 3.80 (s,3H,OCH3),4.42 (s,2H,CH2-N),5.13 (d,2H,J = 6.4 Hz,CH2-NH),8.90 (br s,1H,NH),7.09-7.13 (m,2H,ArH),7.29-7.38 (m,5H,ArH),7.72-7.75 (m,2H,ArH); 13C NMR (100 MHz,CDCl3): δ 46.5 (CH2- NH),48.2 (CH2-N),51.1 (OCH3),97.3,115.1 (d,J = 22.0 Hz),115.7,116.0,121.1 (d,J = 8.0 Hz),125.1 (d,J = 8.0 Hz),127.4,127.5,128.7,134.8 (d,J = 8.0 Hz),139.4,159.8 (d,J = 243.0 Hz),164.3 (C=O),165.4 (C=O).
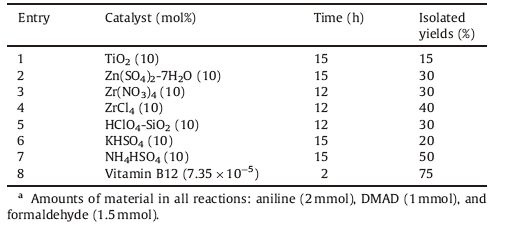
3. Results and discussionThe reaction condition was optimized for the synthesis of 3,4,5- substituted furan-2(5H)-one derivatives,for this purpose the reaction between benzaldehyde,aniline and dimethylacetylendicarboxylatewas chosenas amodel system.The reactionwas initially carried out in different conditions (Table 1). Since we wanted to present a green and environmentally benign protocol for this experiment,we did not test organic solvents under these conditions.
The scope and efficiency of these procedures were explored for the synthesis of a wide variety of substituted 3,4,5-substituted furan-2(5H)-ones (Table 2). Generally,the results were excellent in terms of yield and product purity. A series of aromatic aldehydes and amines were investigated (Table 2,products 4a-o). In all cases,aromatic aldehydes containing electron-donating groups gave shorter times and higher yields than that with electron-withdrawing groups.
|
|
Table 1 Optimization of the reaction conditions for the synthesis of 4a in EtOH.a |
|
|
Table 2 Synthesis of 3,4,5-substituted furan- 2(5H)-ones. |
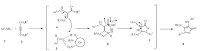
A proposed mechanism for the formation of 4 is shown in Scheme 3. There are many reactive sites in the vitamin B12 molecule that can active carbonyl group (Fig. 1).
|
Download:
|
| Scheme 3.Proposed mechanism for the one-pot three-component synthesis of 3,4,5-substituted furan-2(5H)-ones in the presence of vitamin B12 as green catalyst. | |
Next,the reaction condition was optimized for the synthesis of N-aryl-3aminodihydropyrrol-2-one-4-carboxylates. Formaldehyde,aniline,and dimethyl acetylenedicarboxylate were chosen as model compounds. The reaction was initially carried out in different condition (Table 3). As can be seen in Table 3,catalytic amount of vitamin B12 (7.35 × 10-5 mol%) was found to be the most effective catalyst for the reaction at room temperature.
|
|
Table 3 Optimization of the reaction conditions for the synthesis of 9a in EtOH.a |
To demonstrate the utility and generality of this method,the various substituted anilines,dimethyl or diethyl acetylenedicarboxylates and formaldehyde were employed successfully to generate the desired N-aryl-3-aminodihydropyrrol-2-one-4-carboxylates 9a-h (Table 4). Encouraged by these results,different polyfunctionalized dihydropyrrol-2-ones 9i-p were synthesized using two different amines. Aliphatic amines,such as benzyl amine,1- (pyridin-2-yl)methanamine and n-butyl amine,were reacted with dialkylacetylene-dicarboxylates,anilines and formaldehyde to produce the corresponding products in good to high yields.
|
|
Table 4 Synthesis of N-aryl-3-aminodihydropyrrol-2-one-4-carboxylates. |
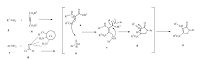
On the basis of the above experimental results,together with the related reports,a proposed reaction mechanism for this onepot,four-component hetero-annulations is illustrated in Scheme 4.
|
Download:
|
| Scheme 4.Proposed mechanism for the one-pot four-component synthesis of N-aryl-3-aminodihydropyrrol-2-one-4-carboxylates in the presence of vitamin B12 as catalyst. | |
In summary,we report an eco-friendly and straightforward one-pot condensation for the synthesis of 3,4,5-trisubstituted furan-2(5H)-ones and N-aryl-3-aminodihydropyrrol-2-one-4-carboxylates in the presence of catalytic amount of vitamin B12 as a highly effective,green and homogenous catalyst. Vitamin B12 is clean,safe,non-toxic,and easy access. Moreover,this method has several other advantages such as,high yields,operational simplicity,clean and neutral reaction conditions,which makes it a useful and attractive process for the synthesis of a wide variety of biologically active compounds.
AcknowledgmentWe gratefully acknowledge financial support from the Research Council of the University of Sistan and Baluchestan,Zahedan,Iran.
| [1] | K. Yamada, Cobalt:its role in health and disease, Met. Ions Life Sci. 13(2013) 295-320. |
| [2] | P.G. Lenhert, D.C. Hodgkin, Structure of the 5,6-dimethylbenzimidazolylcobamide coenzyme, Nature 192(1961) 937-938. |
| [3] | D.C. Hodgkin, The X-ray analysis of complicated molecules, Science 150(1965) 979-988. |
| [4] | D. Dolphin, B12, John Wiley & Sons, New York, 1982. |
| [5] | B. Krautler, D. Arigoni, B.T. Golding, Vitamin B12 and B12-Proteins, John Wiley & Sons, New York, 1998. |
| [6] | R. Banerjee, The Chemistry and Biochemistry of B12, Wiley, New York, 1999. |
| [7] | R. Scheffold, S. Abrecht, H.R. Ruf, et al., Vitamin B12-mediated electrochemical reactions in the synthesis of natural products, Pure Appl. Chem. 59(1987) 363-372. |
| [8] | H. Ogoshi, Y. Kikuchi, T. Yamaguchi, H. Toi, Y. Aoyama, Asymmetric induction in the nucleophilic cyclopropane ring cleavage reaction with vitamin B12s, Organometallics 6(1987) 2175-2178. |
| [9] | G. Pattenden, Cobalt mediated radical reactions in organic synthesis, Chem. Soc. Rev. 17(1988) 361-382. |
| [10] | D.A. Baldwin, E.A. Betterton, S.M. Chemaly, J.M. Pratt, The chemistry of vitamin B12, J. Chem. Soc., Dalton Trans.(1985) 1613-1618. |
| [11] | J.E. Baldwin, R.M. Adlington, T.W. Kang, Direct ring expansion of penicillins to 3-exomethylene cephalosporins, Tetrahedron Lett. 48(1991) 7093-7096. |
| [12] | B.P. Branchaud, G.K. Friestad, in:L. Paquette(Ed.), "Vitamin B12" in Encyclopedia of Reagents for Organic Synthesis, Wiley, New York, 1995, pp. 5511-5514. |
| [13] | S. Miao, R.J. Andersen, A.H. Rubrolides, Metabolites of the colonial tunicate Ritterella rubra, J. Org. Chem. 56(1991) 6275-6280. |
| [14] | M. Kotora, E. Negishi, Highly efficient and selective procedures for the synthesis of gamma-alkylidenbutenolides via palladium-catalyzed enzyme coupling and palladium or silver catalyzed lactonization of(Z)-2-en-4-ynoic acids. Synthesis of rub rolides A, C, D, and E, Synthesis 1(1997) 121-128. |
| [15] | M. Pour, M. Spulak, V. Buchta, et al., 3-Phenyl-5-acyloxymethyl-2H,5H-furan-2-ones:synthesis and biological activity of a novel group of potential antifungal drugs, J. Med. Chem. 44(2001) 2701-2706. |
| [16] | E. Lattmann, N. Sattayasai, C.S. Schwalbe, et al., Novel anti-bacterials against MRSA:synthesis of focussed combinatorial libraries of tri-substituted 2(5H)-furanones, Curr. Drug Discov. Technol. 3(2006) 125-134. |
| [17] | V. Weber, P. Coudert, C. Rubat, etal., Novel 4,5-diaryl-3-hydroxy-2(5H)-furanones as anti-oxidants and anti-inflammatory agents, Bioorg. Med. Chem. 10(2002) 1647-1658. |
| [18] | A. El-Tombary, Y. Abdel-Ghany, A. Belal, E.-D. Shams, F. Soliman, Synthesis of some substituted furan-2(5H)-ones and derived quinoxalinones as potential antimicrobial and anti-cancer agents, Med. Chem. Res. 20(2011) 865-876. |
| [19] | E. Lattmann, W.O. Ayuko, D. Kinchinaton, et al., Synthesis and evaluation of 5-arylated 2(5H)-furanones and 2-arylated pyridazin-3(2H)-ones as anti-cancer agents, J. Pharm. Pharmacol. 55(2003) 1259-1265. |
| [20] | J. Wu, Q. Zhu, L. Wang, R. Fathi, Z. Yang, Palladium-catalyzed cross-coupling reactions of 4-tosyl-2(5H)-furanone with boronic acids:a facile and efficient route to generate 4-substituted 2(5H)-furanones, J. Org. Chem. 68(2003) 670-673. |
| [21] | J. Boukouvalas, R.P. Loach, General, regiodefined access to a-substituted butenolides through metal-halogen exchange of 3-bromo-2-silyloxyfurans. Efficient synthesis of an anti-inflammatory gorgonian lipid, J. Org. Chem. 73(2008) 8109-8112. |
| [22] | D. Lee, S.G. Newman, M.S. Taylor, Boron-catalyzed direct Aldol reactions of pyruvic acids, Org. Lett. 11(2009) 5486-5489. |
| [23] | S. Cacchi, G. Fabrizi, A. Goggiamani, A. Sferrazza, Palladium-catalyzed reaction of arenediazonium tetrafluoroborates with methyl 4-hydroxy-2-butenoate:an approach to 4-aryl butenolides and an expeditious synthesis of rubrolide E, Synlett(2009) 1277-1280. |
| [24] | M. Bassetti, A.D. Annibale, A. Fanfoni, F. Minissi, Synthesis of α,β-unsaturated 4,5-disubstituted γ-lactones via ring-closing metathesis catalyzed by the first-generation Grubbs' catalyst, Org. Lett. 7(2005) 1805-1808. |
| [25] | K. Matuso, M. Shindo, Cu(Ⅱ)-catalyzed acylation by thiol esters under neutral conditions:tandem acylation-wittig reaction leading to a one-pot synthesis of butenolides, Org. Lett. 12(2010) 5346-5349. |
| [26] | Y. Liu, F. Song, S. Guo, Cleavage of a carbon-carbon triple bond via gold-catalyzed cascade cyclization/oxidative cleavage reactions of(z)-enynols with molecular oxygen, J. Am. Chem. Soc. 128(2006) 11332-11333. |
| [27] | D. Tejedor, A. Santos-Expósito, F. García-Tellado, A convenient entry to 5-(sp2)-substituted and 5,5-disubstituted tetronic acids, Synlett(2006) 1607-1609. |
| [28] | S. Narayana Murthy, B. Madhav, A. Vijay Kumar, K. Rama Rao, Y.V.D. Nageswar, Facile and efficient synthesis of 3,4,5-substituted furan-2(5H)-ones by using bcyclodextrin as reusable catalyst, Tetrahedron 65(2009) 5251-5256. |
| [29] | L. Nagarapu, U.N. Kumar, P. Upendra, R. Bantu, Simple, convenient method for the synthesis of substituted furan-2(5H)-one derivatives using Tin(Ⅱ) chloride, Synth. Commun. 42(2012) 2139-2148. |
| [30] | W.J. Bai, S.K. Jakson, T.R.R. Pettus, Mild construction of 3-methyl tetramic acids enabling a formal synthesis of Palau'imide, Org. Lett. 14(2012) 3862-3865. |
| [31] | M. Aginagalde, T. Bello, C. Masdeu, et al., Formation of γ-oxoacids and 1H-pyrrol-2(5H)-ones from α,β-unsaturated ketones and ethyl nitroacetate, J. Org. Chem. 75(2010) 7435-7438. |
| [32] | H. Anaraki-Ardakani, M. Noei, A. Tabarzad, Facile synthesis of N-(arylsulfonyl)-4-ethoxy-5-oxo-2,5-dihydro-1H-pyrolle-2,3-dicarboxylates by one-pot three-component reaction, Chin. Chem. Lett. 23(2012) 45-48. |
| [33] | L. Ettlinger, E. Gäuemann, R. Hütter, et al., Metabolites of actinomycetes 17. Mitteilung Holomycin, Helv. Chim. Acta 42(1959) 563-566. |
| [34] | H. Shiozawa, S. Takahashi, Configurational studies on thiomarinol, J. Antibiot. 47(1994) 851-853. |
| [35] | S.B. Singh, M.A. Goetz, E.T. Jones, et al., Oteromycin:a novel antagonist of endothelin receptor, J. Org. Chem. 60(1995) 7040-7042. |
| [36] | H. He, H.Y. Yang, R. Bigelis, E.H. Solum, M. Greenstein, Pyrrocidines A and B, new antibiotics produced by a filamentous fungus, Tetrahedron Lett. 43(2002) 1633-1636. |
| [37] | A.J. Clark, C.P. Dell, J.M. McDonagh, J. Geden, P. Mawdsley, Oxidative 5-endo cyclization of enamides mediated by ceric ammonium nitrate, Org. Lett. 5(2003) 2063-2066. |
| [38] | J. Chen, P.Q. Huang, Y. Queneau, Enantioselective synthesis of the R-enantiomer of the feeding deterrent(S)-ypaoamide, J. Org. Chem. 74(2009) 7457-7463. |
| [39] | A. Raghuraman, E. Ko, L.M. Perez, T.R. Ioerger, K. Burgess, Pyrrolinone-pyrrolidine oligomers as universal peptidomimetics, J. Am. Chem. Soc. 133(2011) 12350-12353. |
| [40] | T. Kawasuji, M. Fuji, T. Yoshinaga, et al., 3-Hydroxy-1,5-dihydro-pyrrol-2-one derivatives as advanced inhibitors of HIV integrase, Bioorg. Med. Chem. 15(2007) 5487-5492. |
| [41] | L. Zhang, Y. Tan, N.X. Wang, et al., Design, syntheses and 3D-QSAR studies of novel N-phenyl pyrrolidin-2-ones and N-phenyl-1H-pyrrol-2-ones as protoporphyrinogen oxidase inhibitors, Bioorg. Med. Chem. 18(2010) 7948-7956. |
| [42] | Y. Mizushina, S. Kobayashi, K. Kuramochi, et al., Epolactaene, a novel neuritogenic compound in human neuroblastoma cells, selectively inhibits the activities of mammalian DNA polymerases and human DNA topoisomerase Ⅱ, Biochem. Biophys. Res. Commun. 273(2000) 784-788. |
| [43] | Q. Zhu, L. Gao, Z. Chen, et al., A novel class of small-molecule caspase-3 inhibitors prepared by multicomponent reactions, Eur. J. Med. Chem. 54(2012) 232-238. |
| [44] | B. Li, M.P.A. Lyle, G. Chen, et al., Substituted 6-amino-4H-[1,2] dithiolo[4,3-b]pyrrol-5-ones:synthesis, structure-activity relationships, and cytotoxic activity on selected human cancer cell lines, Bioorg. Med. Chem. 15(2007) 4601-4608. |
| [45] | A.S. Demir, F. Aydigan, I.M. Akhmedov, The synthesis of chiral 5-methylene pyrrol-2(5H)-ones via photooxygenation of homochiral 2-methylpyrrole derivatives, Tetrahedron:Asymmetry 13(2002) 601-605. |
| [46] | T.R.K. Reddy, C.L.X. Guo, X. Myrvang, H.K.P.M. Fischer, L.V. Dekker, Design, synthesis, and structure-activity relationship exploration of 1-substituted 4-Aroyl-3-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one analogues as inhibitors of the annexin A2-S100A10 protein interaction, J. Med. Chem. 54(2011) 2080-2094. |
| [47] | Q. Zhu, H. Jiang, J. Li, et al., Concise and versatile multicomponent synthesis of multisubstituted polyfunctional dihydropyrroles, J. Comb. Chem. 11(2009) 685-696. |
| [48] | A.T. Khan, A. Ghosh, M.D.M. Khan, One-pot four-component domino reaction for the synthesis of substituted dihydro-2-oxypyrrole catalyzed by molecular iodine, Tetrahedron Lett. 53(2012) 2622-2626. |
| [49] | H. Gao, J. Sun, C.G. Yan, Synthesis of functionalized 2-pyrrolidinones via domino reactions of arylamines, ethyl glyoxylate and acetylenedicarboxylates, Tetrahedron 69(2013) 589-594. |
| [50] | S. Rana, M. Brown, A. Dutta, A. Bhaumik, C. Mukhopadhyay, Site-selective multicomponent synthesis of densely substituted 2-oxo dihydropyrroles catalyzed by clean, reusable, and heterogeneous TiO2 nanopowder, Tetrahedron Lett. 54(2013) 1371-1379. |
| [51] | L. Lv, S. Zheng, X. Cai, et al., Development of four-component synthesis of tetraand pentasubstituted polyfunctional dihydropyrroles:free permutation and combination of aromatic and aliphatic amines, ACS Comb. Sci. 15(2013) 183-192. |
| [52] | R. Doostmohammadi, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, An efficient one-pot multi-component synthesis of 3,4,5-substituted furan-2(5H)-ones catalyzed by tetra-n-butylammonium bisulfate, Chin. Chem. Lett. 24(2013) 901-903. |
| [53] | M. Kangani, M.T. Maghsoodlou, N. Hazeri, Synthesis of pyrrole and furan derivatives in the presence of lactic acid as a catalyst, J. Saud. Chem. Soc.(2015), http://dx.doi.org/10.1016/j.jscs.2015.03.002. |
| [54] | M.T. Maghsoodlou, S.M. Habibi-Khorasani, Z. Shahkarami, N. Maleki, M. Rostamizadeh, An efficient synthesis of 2,2'-arylmethylene bis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) and 1,8-dioxooctahydroxanthenes using ZnO and ZnO-acetyl chloride, Chin. Chem. Lett. 21(2010) 686-689. |
| [55] | Z. Vafajoo, N. Hazeri, M.T. Maghsoodlou, H. Veisi, Electro-catalyzed multicomponent transformation of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one to 1,4-dihydropyrano[2,3-c]pyrazole derivatives in green medium, Chin. Chem. Lett.(2015), http://dx.doi.org/10.1016/j.cclet.2015.04.016. |
 2016, Vol.27
2016, Vol.27