b State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China;
c Key Laboratory of Structure-Based Drug Design & Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang 110016, China;
d Institute of Traditional Chinese Medicine & Natural Products, College of Pharmacy, Jinan University, Guangzhou 510632, China
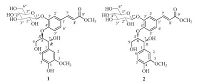
Matteuccia struthiopteris (L.) Todar. is an edible plant and traditional medicine,which is widely distributed in the temperate regions of the northern hemisphere [1, 2, 3, 4]. The rhizomes of M. struthiopteris are used as both traditional Chinese and folk medicines with the efficacy of heat-clearing and detoxifying,insecticidal,hemostasis and anti-influenza virus [2]. The tender leaves of M. struthiopteris are well-known wild vegetable in northeast of China,Japan,United States and Canada,which can be eaten directly or processed by canning,freezing and salting [3, 4]. Some flavonoids,phenolics,stilbenes and steroids have been identified from the rhizomes of M. struthiopteris before [3, 5, 6, 7, 8]. Our previous study on the rhizomes of M. struthiopteris led to the isolation of some flavonoids possessing inhibitory activity against the H1N1 influenza virus [9]. However,no investigation has been reported on the chemical constituents and bioactivity from the aerial parts of M. struthiopteris. Our systematic study on the 60% EtOH extract of the aerial parts of M. struthiopteris led to the isolation of two new 8-O-40 neolignan glycosides,matteustruthiosides A (1) and B (2) (Fig. 1). This paper deals with the isolation,structural elucidation and anti-influenza virus (H1N1) activity of two new compounds.
|
Download:
|
| Fig. 1.Chemical structures of compounds 1 and 2. | |
The aerial parts of M. struthiopteris were collected from Liaoning province,China,in September 2011,and taxonomically identified by Prof. Jin-Cai Lu,College of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University,China,where a herbarium specimen is deposited (ZYFMS-2011).
2.2. Extraction and isolationThe aerial parts of M. struthiopteris (8.8 kg) were extracted with EtOH-H2O (60:40,v/v) twice,under condition of reflux,2 h each time,and then the combined extracts were concentrated by evaporation to yield a brownish extract (800 g),which was applied to a Diaion HP-20 macroporous adsorptive resins column eluted with EtOH-H2O in gradient. The 50% EtOH-H2O eluate (C,18 g) was then subjected to silica gel column chromatography (CC),eluted with a CH2Cl2-MeOH gradient to give 10 fractions (C1-10). Fraction C7 (CH2Cl2-MeOH 93:7 eluate,2.8 g) was subjected to ODS CC,eluted with a MeOH-H2O gradient,to yield 9 subfractions (C71-79). Subfraction C77 (MeOH-H2O 40:60 eluate,200.0 mg) was purified by semi-preparative HPLC with MeOH-H2O (30:70,v/v) to afford 1 (14.3 mg) and 2 (8.3 mg).
2.3. Matteustruthioside AYellowish solid; [α]D25 -1.62 (c 0.5,MeOH). UV (MeOH) λmax (log ε): 235 (4.29),295 (4.25),317 (4.25) nm; CD (MeOH): 237 (Δε -0.48); IR (KBr,cm-1): vmax 3396,1701,1601,1509; HR-ESI-MS m/z 575.1742 [M+Na]+,calcd. for C26H32O13Na 575.1741; 1H NMR and 13C NMR data,see Table 1.
|
|
Table 1 1H NMR and 13C NMR data for compounds 1 and 2.a |
Yellowish solid; [α]D25 -4.48 (c 0.5,MeOH). UV (MeOH) λmax (log ε): 235 (4.20),292 (4.16),318 (4.17) nm; CD (MeOH): 237 (Δε +0.99); IR (KBr,cm-1): nmax 3396,1699,1601,1509; HR-ESI-MS m/z 575.1744 [M+Na]+,calcd. for C26H32O13Na 575.1741; 1H NMR and 13C NMR data,see Table 1.
2.5. Sugar analysisThe absolute configuration of the sugar moiety was determined by the method of Tanaka et al. [9]. Compounds 1 and 2 both afford D-glucose (tR = 19.5 min).
3. Results and discussionCompound 1 was obtained as yellowish solid,[α]D25 -1.62 (c 0.5,MeOH). The molecular formula was determined as C26H32O13 by HR-ESI-MS at m/z 575.1742 (calcd. for C26H32O13Na 575.1741) (Fig. S1 in Supporting information). Its IR spectrum (Fig. S3 in Supporting information) displayed absorption bands corresponding to OH group(s) (3396 cm-1),carbonyl group(s) (1701 cm-1),and aromatic ring(s) (1601 and 1509 cm-1). The 1H NMR spectrum of 1 (Fig. S4 in Supporting information) showed six aromatic proton signals due to two 1,3,4-trisubstituted benzene rings at d 7.48 (d,1H,J = 1.8 Hz,H-2'),7.19 (dd,1H,J = 8.5,1.8 Hz,H-6'),6.97 (d,1H,J = 8.5 Hz,H-5'),and 7.03 (d,1H,J = 1.6 Hz,H-2),6.90 (dd,1H,J = 8.1,1.6 Hz,H-6),6.75 (d,1H,J = 8.1 Hz,H-5),two transolefinic protons at δ 7.59 (d,1H,J = 16.0 Hz,H-7') and 6.40 (d,1H,J = 16.0 Hz,H-8'),two methoxy group protons at δ 3.81 (3H,s) and 3.76 (3H,s),and a sugar moiety including an anomeric proton signal at δ 4.81 (d,1H,J = 7.6 Hz,H-1'') together with five proton signals at δ 3.52 (m,1H,H-2''),3.46 (m,1H,H-3''),3.37 (m,1H,H-4''),3.38 (m,1H,H-5''),3.87 (m,1H,H-6a'') and 3.69 (m,1H,H-6b''). Remaining resonances at δ 4.87 (d,1H,J = 7.2 Hz,H-7),4.54 (m,1H,H-8),3.91 (dd,1H,J = 12.2,5.8 Hz,H-9a),3.78 (dd,1H,J = 12.2,2.9 Hz,H-9b) established the occurrence of a glyceryl unit. The 13C NMR spectrum of 1 (Fig. S5 in Supporting information) in combination with the DEPT and HSQC spectra (Figs. S6 and S8 in Supporting information) indicated the presence of 26 carbons. Aside from a hexose moiety and two methoxy groups,the remaining 18 carbon signals and the HMBC correlations of H-7 at δ 4.87 with C-1,C-2,C-6,C-8 and C-9,and of H-7' at δ 7.59 with C-1',C-2',C-6',C-8' and C-9' confirmed the presence of two phenylpropanoid units. HMBC correlation from δ 4.54 (H-8) to d 152.3 (C-4') suggested 1 was an 8-O-4' neolignan.
After acid hydrolysis and derivatization of 1 with the reported method [10],HPLC analysis revealed the presence of D-glucose. Additionally,the β-configuration was established due to the coupling constant (7.6 Hz) of the anomeric proton. Location of the glucose was determined by the HMBC correlation from H-1'' to C-3'. Likewise,the methoxy groups were positioned at C-3 and C-9',respectively.
The large coupling constant (J = 7.2 Hz) between H-7 and H-8 suggested that the relative configuration of H-7 and H-8 was threo by comparison with the coupling constant (J7,8 > 7.0 Hz for threo and J7,8 < 6.0 Hz for erythro) reported [11]. The 8R absolute configuration was determined by the CD spectrum of 1 (Fig. 2),which displaying a negative Cotton effect at 237 nm [12]. On the basis of the above evidence,the structure of 1 was determined to be (7R,8R)-threo-(E)-4,7,9-trihydroxy-3,90-dimethoxy-70-en-90- oxo-8-O-40-neolignan-3'-O-β-D-glucopyranoside,designated as matteustruthioside A.

|
Download:
|
| Fig. 2.CD spectra of compounds 1 and 2. | |
Compound 2 was isolated as yellowish solid,[α]D25 -4.48 (c 0.5,MeOH). Positive HR-ESI-MS data indicated a quasimolecular ion peak at m/z 575.1744 [M+Na]+ (Fig. S10 in Supporting information),corresponding to the molecular formula C26H32O13. Its 1HNMR and 13C NMR spectroscopic data (Figs. S13 and S14 in Supporting information) were very similar to those of 1. Further analyses of the HSQC and HMBC data (Figs. S17 and S18 in Supporting information) of 2 deduced an identical planar structure to that of 1,suggesting that 2 was a diastereoisomer of 1. The sugar unit was determined to be β-D-glucose by acid hydrolysis and the coupling constant (7.2 Hz) of the anomeric proton. The small coupling constant (J = 4.8 Hz) between H-7 and H-8 showed that 2 has erythro configuration [11]. The positive CD effect at 237 nm revealed a 7R,8S-configuration of 2 (Fig. 2) [12]. Therefore,2 was elucidated as (7R,8S)-erythro-(E)-4,7,9-trihydroxy-3,90-dimethoxy- 70-en-90-oxo-8-O-40-neolignan-30-O-β-D-glucopyranoside,named as matteustruthioside B.
Compounds 1 and 2 were evaluated for their anti-influenza virus (H1N1) activity by neuraminidase inhibition assay. Alamar- Blue assay was used to measure cell viability in parallel. The two compounds showed low cytotoxicity,but unfortunately were inactive in neuraminidase inhibition assay.
4. ConclusionPhytochemical investigation on the 60% EtOH extract of the aerial parts of M. struthiopteris resulted in the isolation of two new 8-O-40 neolignan glycosides,assigned as matteustruthiosides A (1) and B (2). Compounds 1 and 2 were found to be inactive against neuraminidase from H1N1 influenza virus in vitro.
AcknowledgmentsThe authors thank Prof. Jin-Cai Lu,at College of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University,China for identification of the plant materials. We are grateful to Institute of Traditional Chinese Medicine & Natural Products,College of Pharmacy,Jinan University for measuring the optical rotation data and HR-ESI-MS spectra. This work was financially supported by State Key Laboratory of Bioactive Substance and Function of Natural Medicines (No. GTZK201304) and the Key Projects of the National Science and Technology Pillar Program (No. 2012BAI30B02).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.08.016.
| [1] | Flora of China Editorial Committee, Flora Reipublicae Popularis Sinicae, 4, Science Publishers, Beijing, 1999, pp. 160-162. |
| [2] | Nanjing University of Chinese, Medicine, Dictionary of traditional Chinese Herbal Medicine, 2nd ed., Shanghai Scientific and Technologic Publishers, Shanghai, 2006, pp. 2118-2119. |
| [3] | T. Kimura, M. Suzuki, M. Takenaka, et al., l-O-Caffeoylhomoserine from Matteuccia struthiopteris, Phytochemistry 65(2004) 423-426. |
| [4] | A.A. Bushway, D.V. Serreze, D.F. McGann, et al., Effect of processing method and storage time on the nutrient composition of Fiddlehead Greens, J. Food Sci. 50(1985) 1491-1492. |
| [5] | D. Zhang, S.B. Li, L. Yang, et al., Two new C-methyl flavanones from the rhizomes and frond bases of Matteuccia struthiopteris, J. Asian Nat. Prod. Res. 15(2013) 1163-1167. |
| [6] | L. Yang, M.Y. Wang, Y.Y. Zhao, et al., Studies on chemical constituents in rhizome of Matteuccia struthiopteris, Chin. J. Chin. Mater. Med. 29(2004) 647-649. |
| [7] | L. Yang, M.Y. Wang, Y.Y. Zhao, et al., Chemical constituents of rhizome of Matteuccia struthiopteris, Acta Pharmacol. Sin. 40(2005) 252-254. |
| [8] | D. Zhang, L. Yang, M.H. Fu, et al., Studies on chemical constituents of rhizome of Matteuccia struthiopteris, Chin. J. Chin. Mater. Med. 33(2008) 1703-1705. |
| [9] | B. Li, Y. Ni, L.J. Zhu, et al., Flavonoids from Matteuccia struthiopteris and their antiinfluenza virus(H1N1) activity, J. Nat. Prod. 78(2015) 987-995. |
| [10] | T. Tanaka, T. Nakashima, T. Ueda, et al., Facile discrimination of aldose enantiomers by Reversed-Phase HPLC, Chem. Pharm. Bull. 55(2007) 899-901. |
| [11] | X. Cheng, J. Qin, Q. Zeng, et al., Taraxasterane-type triterpene and neolignans from Geum japonicum Thunb. var. chinense F. Bolle, Planta Med. 77(2011) 2061-2065. |
| [12] | M. Gan, Y. Zhang, S. Lin, et al., Glycosides from the root of Iodes cirrhosa, J. Nat. Prod. 71(2008) 647-654. |
 2016, Vol.27
2016, Vol.27