Friedel-Crafts reactions are widely considered as one of the most fundamental C-C bond-forming reactions in organic synthesis [1]. Among the aromatic systems suitable for Friedel-Crafts reactions,indoles have received much attention. The preparation and functionalization of indoles remain to be a fascinating subject in organic synthesis due to their vast existence in biologically interesting systems,natural products,and drug-like molecules [2].
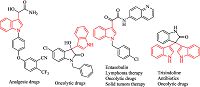
The C3 functionalized indoles,for instance,as a featured heterocyclic nucleus in a number of natural products and drug molecules,are good examples (Fig. 1) [3]. In addition,1-hydroxy-1- (1H-indol-3-yl)propan-2-one is also an emerging new scaffold for drug discovery with a broad spectrum of biological activities including analgesic,antiviral,antibacterial,antitubercular,antiinflammatory,antiangiogenic,antifungal,anticonvulsant and cancer chemotherapeutical activities [4]. Notably,these derivatives have been served as potential building blocks for complex natural product syntheses [5]. Likewise,the C3 functionalized bisindole compounds have also received a considerable amount of attention because of their good bioactivity [6]. Therefore,efforts have been made to synthesize C3 substituted indoles through either the construction or modification of indole rings. For example,Deng’s group established a Friedel-Crafts reaction of indoles with ethyl glyoxylate catalyzed by bifunctional cinchona alkaloids [7],Zhu’s group reported the combination of a weak lewis acid (LiCl) and a weak brønsted acid (hexafluoroisopropanol,HFIP) promoted the Friedel-Crafts reaction of electron-rich aromatic compounds with ethyl glyoxylate efficiently [8],Feng and co-workers reported a highly efficient asymmetric synthesis of 3-indolyl(hydroxy) acetates via the Friedel-Crafts alkylation of indoles [9].
|
Download:
|
| Fig. 1. Selected biologically active molecules containing a C3 functionalized indole moiety. | |
Although C3 substituted indole compounds,especially the 1-hydroxy-1-(1H-indol-3-yl)propan-2-one derivatives,show good bioactivity,the synthesis and application of 2-hydroxy-2-(1Hindol- 3-yl)-N-substituted acetamide and the bisindole compounds have not been systematically studied to our best knowledge. Thus,we report here our recent study on the synthesis and applications of the 2-hydroxy-2-(1H-indol-3-yl)-N-substituted acetamides and the bisindole compounds.
2. Experimental1H NMR and 13CNMR spectraweremeasured on a Bruker Avance 400 or 600 MHz NMR spectrometer usingDMSO-d6 as a solvent and recorded in ppm relative to an internal tetramethylsilane standard. Thin layer chromatography (TLC) characterization was performed with precoated silica gel GF254 (0.2 mm),while column chromatography characterization was performed with silica gel (100-200 mesh). Melting points were measured with a YRT-3 melting point apparatus (Shantou Keyi Instrument& Equipment Co.,Ltd.,Shantou,China). High resolution mass spectroscopy data of the products were collected on a Waters Micromass GCT or a Bruker Apex IV FTMS instrument. All the N-substituted glyoxylamides 1 were prepared according to the reported procedures [10]. Indoles 1 were purchased fromASTATECH except for ethyl 2-((1-methyl-1H-indol- 6-yl)oxy)acetate,which was synthesized according to the literature procedures [11]. General chemicals were purchased from commercial suppliers and used without further purification.
2.1. General reaction procedure for the regioselective Friedel-Crafts hydroxyalkylation of N-substituted glyoxylamide with indolesN-Substituted glyoxylamide (1.0 mmol),indoles (1.1 mmol),and 20 mmol% FeSO4 were added to 6 mL of C2H5OH and then the mixture was stirred at room temperature for 0.5 h. After the completion of the reaction (monitored by TLC analysis),the mixture was diluted with water and extracted with ethyl acetate (20 mL × 4). The organic layer was washed with saturated brine,dried over anhydrous sodium sulfate and the solvent was evaporated to dryness. The crude residue was purified by flash chromatography on silica (dichloromethane/methanol = 80/1) to afford pure 2-hydroxy-2-(1H-indol-3-yl)-N-substituted acetamides 3 (54%-93% yield).
N-Substituted glyoxylamide (1.0 mmol),indoles (2.0 mmol),and 10 mmol% FeCl3 were added to 6 mL of C2H5OH and then the mixture was stirred at room temperature for 0.5 h. After the completion of the reaction (monitored by TLC analysis),the mixture was diluted with water and extracted with ethyl acetate (20 mL × 3). The organic layer was washed with saturated brine,dried over anhydrous sodium sulfate and the solvent was evaporated to dryness. The crude residue was purified by flash chromatography on silica (dichloromethane/methanol = 100/1) to afford pure 2,2-di(1H-indol-3-yl)-N-phenylacetamide derivatives 4 (71%-92% yield).
3. Results and discussionIn the present study,our investigation started with screening Lewis acid catalysts for the Friedel-Crafts hydroxyalkylation of Nphenyl glyoxylamide (1a) with indole (2a). When 1a was treated with 2a in the presence of aluminium chloride,which is a commonly used Lewis acid,the desired 2-hydroxy-2-(1H-indol-3- yl)-N-phenylacetamide 3a was isolated in 48% yield (Table 1,entry 1). And a side product was found. Separation and confirmation of this side product suggested it was the bisindole product 4a,as shown in Table 1. The product 3a and its derivatives could be a potentially useful intermediate for the synthesis of A2B AR modulator and antitumor agents [12],while the side product 4a could be treated as an open chain analogue of trisindoline,which may have good antibiotic and oncolytic bioactivity [13]. However,poor selectivity was found when a mixture of N-phenyl glyoxylamide with indole was treated with aluminium chloride. We hypothesized that the reaction selectivity could be controlled using an appropriate Lewis acid catalyst. Therefore,we were interested in looking for an efficient and highly selective Lewis acid as the catalyst in the synthesis of 3a and 4a,respectively.
At the beginning of our study,the screening of reaction conditions was focused on a variety of reaction parameters using a model reaction of N-phenyl glyoxylamide (1a) with indole (2a). The results are summarized in Table 1. Various Lewis acids such as AlCl3,SnCl4,CoCl2,MgCl2,CrCl3,ZnCl2,CuCl2,FeCl3,FeSO4,MnCl2,NiCl2,Ce(NO3)3,In(CF3SO3)3,AgSbF6 and K4[Fe(CN)6] were used. Among these catalysts,FeCl3 and FeSO4 exhibited the highest selectivity (Table 1,entries 1 and 1 ). In addition,the screening of different Lewis acids (Table 1,entries 1-15) led to the discovery that FeSO4 and FeCl3 were the most effective catalysts,forming the desired products 3a (Table 1,entry 9,83% yield) and 4a (Table 1,entry 8,92% yield) in a high level of yield respectively. Furthermore,other iron(II) and iron(III) halides such as FeCl2 and FeBr3 were used,forming the desired products 3a (Table 1,entry 16,81% yield) and 4a (Table 1,entry 17,90% yield) in a similarly high yields as those achieved by the reactions catalyzed by FeSO4 and FeCl3 respectively. In terms of the optimal amount of FeSO4 needed in the model reaction,it was found that the yield of the desired product 3a increased to 93% (Table 1,entry 18) when 20 mol% of FeSO4 was used,and the yield for the desired product 3a increased slightly while the yield for 4a increased significantly when 100 mol% of FeSO4 was used (Table 1,entry 19). Therefore,using 20 mol% FeSO4 was found to be more effective. Among various solvents examined,C2H5OH proved to be the best choice,while others such as THF,CH3CN,DMSO,dioxane,acetone,toluene,CH2Cl2,CH3COOC2H5 were less effective (Table 1,entries 1 and 20 - 27 ). We did not find the desired increment of the formation of the product 3a while 4a was enriched slightly when the reaction time was extended to 24 h (Table 1,entry 28). Further investigation indicated that temperature was important for this transformation. An excellent yield was obtained when the reaction was carried out at 25 ℃ (Table 1,entry 18). However,when the temperature increased to 50 ℃ and 80 ℃,the yield of the desired product 3a dropped to 66% and 41% (Table 1,entries 29,30). And when the reaction was conducted to 0 ℃ (Table 1,entry 31),the yield of 3a dropped to 84%. Therefore,in concerns of catalytic selectivity and level of yield,we could selectively obtain the product 3a in 93% yield using 20 mol% of FeSO4 as a catalyst and C2H5OH as the solvent and 4a in 92% yield using 10 mol% of FeCl3 as a catalyst and C2H5OH as the solvent,respectively.
|
|
Table 1 Screening studies for the regioelective Friedel-Crafts hydroxyalkylation of N-phenyl glyoxylamide with indole.a |
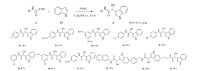
With optimized conditions in hand,a series of regioselective Friedel-Crafts hydroxyalkylation of N-substituted glyoxylamide with indole 2a were thus carried out. The results were summarized in Fig. 2. Among various tested substrates,a variety of N-phenyl glyoxylamide derivatives bearing either electron-donating groups such as methyl and methoxy,or electron-withdrawing groups such as chloro,bromo and nitro,were well tolerated during the course of the reaction providing the desired products 3a-3l in moderate to good yields.
|
Download:
|
| Fig. 2.Substrate scope in hydroxyalkylation of N-substituted glyoxylamide with indole using FeSO4 as the catalyst. Reaction conditions: N-substituted glyoxylamide (1,1.0 mmol),indole (2a,1.1 mmol),and 20 mmol% FeSO4 in C2H5OH were stirred at room temperature for 0.5 h. | |
Moderate yields of N-phenyl glyoxylamide derivatives containing electron-donating groups linked to the benzene ring were obtained (3b-3d),while N-phenyl glyoxylamide derivatives containing electron-withdrawing groups linked to the benzene ring resulted in good yields (3e-3i). These results suggested that the reactivity of the N-phenyl glyoxylamide derivatives was influenced by electronic property of the groups linked to the benzene ring. The reactivity of N-phenyl glyoxylamide derivatives could be reduced by an electron-donating group linked to the benzene ring.
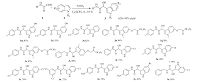
To further define the scope of this transformation,a wide range of N-phenyl glyoxylamide derivatives and indoles containing different substituents at the 1-,2-,5- or 6-positions were reacted under the optimized reaction conditions (Fig. 3). Good to excellent yields of indoles having electron-donating groups were obtained in most cases (3m-3s). In contrast,indoles substituted with electronwithdrawing groups resulted in only moderate yields (3t-3z). These results suggested that the reactivity of the indole substrates was remarkably influenced by the electronic property of the substituent of the indole. The reactivity of indole substrate could be reduced by an electron-withdrawing group linked to indole. Therefore,these results clearly demonstrated that ferrous sulfate served as a useful Lewis acid catalyst for the N-substituted glyoxylamide and indoles to produce the corresponding 2- hydroxy-2-(1H-indol-3-yl)-N-substituted acetamide.
|
Download:
|
| Fig. 3.N-Substituted glyoxylamide (1,1.0 mmol),indoles (2,1.1 mmol),and 20 mmol% FeSO4 in C2H5OH were stirred at room temperature for 0.5 h. | |
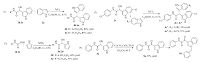
Furthermore,with optimized conditions in hand,a series of bisindole compounds resulting from the regioselective Friedel- Crafts hydroxyalkylation were carried out. The results were summarized in Fig. 4. Among various tested substrates,N-phenyl glyoxylamide derivatives had either electron-donating (4b' ,4b'' ,4c,4c' ) or electron-withdrawing substituents (4e,4e' ,4i,4i' ) on the benzene ring could be tolerated and the corresponding products were isolated in good yields. However,the yields of indoles containing electron-withdrawing groups at 5- or 6-position (4b' ,4b'' ,4c' ,4i' ) were slightly lower than the others. The result suggested that the reactivity of the regioselective Friedel-Crafts hydroxyalkylation could be reduced by an electron-withdrawing group linked to the 5- or 6-position of indole.

|
Download:
|
| Fig. 4.Substrate scope in reactions of N-substituted glyoxylamide with indole using FeCl3 as the catalyst. Reaction conditions: N-substituted glyoxylamide (1,1.0 mmol),indoles (2,2.0 mmol),and 10 mmol% FeCl3 in C2H5OH were stirred at room temperature for 0.5 h. | |
Moreover,product 1 could also be transformed to 1 in the presence of a catalytic amount of ferric trichloride at room temperature. For example,3b and 3e were reacted with indole in the presence of FeCl3 at room temperature to afford the corresponding bisindole products 4b and 4e in high yields. Therefore,a series of different-substituted bisindole products could be obtained in the presence of 3. For example,3v was reacted with 5-hydroxyindole and 6-cyanoindole to afford the corresponding different-substituted bisindole products 4v' and 4v'' . And when 1b and 1e were treated with pyrrole under the optimized condition,the corresponding product 3b' and 3e' were obtained (Scheme 1).
|
Download:
|
| Scheme 1.Applications of the regioselective Friedel-Crafts hydroxyalkylation | |
Then the applications of this newLewis acid-catalyzed method in the synthesis of biologically active compounds were studied. The resulting 2-hydroxy-2-(1H-indol-3-yl)-N-substituted acetamide can be transformed to various biologically active compounds. For example,3a could readily be oxidized to 5a [14]. which is an important building block for the synthesis of the A2B AR modulator 5a' . What is more,as trisindoline and other bisindole compounds have good antibiotic and oncolytic bioactivity,their open chain analogue 4a and derivatives that have the similar double indoles might potentially have good antibiotic and oncolytic bioactivity,which is under investigation (Scheme 1).
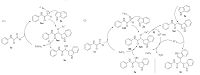
Therefore,a plausible reaction mechanism for the Lewis acidcatalyzed regioselective Friedel-Crafts hydroxyalkylation is outlined in Scheme 2,using the hydroxyalkylation of N-phenyl glyoxylamide with indole as a representative. In the first step of ferrous sulfate-catalyzed reaction,the ferrous ion might coordinate with N-phenyl glyoxylamide 1a. Then indole could attack the activated carbonyl to form the intermediate 3aa. Further,intermediate 3aa may follow pathway I to provide the product 3a and release the ferrous sulfate for the catalytic cycle. In the first step of ferric trichloride-catalyzed reaction,the ferric ion might coordinate with N-phenyl glyoxylamide 1a,then indole could attack the activated carbonyl to form the intermediate 3ab. In the following steps,intermediate 3ab may follow pathway II to give the bisindole product 4a and release the ferric trichloride for the catalytic cycle [15]. Furthermore,3a could coordinate with ferric ion and follow pathway III to form intermediate 3ab and then follow pathway II to give the bisindole product 4a. The regioselective Friedel-Crafts hydroxyalkylation could be catalyzed by ferrous sulfate and ferric trichloride to form the 3a and 4a in a high yield respectively for the reason that the intermediate 3aa is probably much less stable than the intermediate 3ab. 3aa transforms to the product 3a rapidly,while 3ab transforms to the stable intermediate 3ac and then forms the product 4a.
|
Download:
|
| Scheme 2. A proposed mechanism accounting for the formation of 3a and 4a. | |
In this study,a Lewis acid-catalyzed regioselective Friedel- Crafts hydroxyalkylation of N-substituted glyoxylamides with various substituted indoles using the readily available reagent FeSO4 and FeCl3 are successfully developed. The reactions are demonstrated to have high yield and selectivity under mild conditions,and are compatible with many functional groups. With these demonstrated advantages,we anticipate that such reactions can be a straightforward and practical way to prepare various 2- hydroxy-2-(1H-indol-3-yl)-N-substituted acetamide and 2,2- di(1H-indol-3-yl)-N-phenylacetamide derivatives.
AcknowledgmentWe sincerely thank the National Natural Science Foundation of China (Nos. 21472130,81373259) for financial support of this study.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.09.023.
| [1] | For reviews of Friedel-Crafts reactions, see:(a) GA. Olah, Crafts Chemistry, Wiley-Interscience, New York, 1973;(b) R.M. Roberts, A.A. Khalaf, Friedel-Crafts Alkylation Chemistry. A Century of Discovery, M. Dekker, New York, 1984;(c) H. Heaney, in:B.M. Trost, I. Fleming(Eds.), Comprehensive organic synthesis, Vol. 2, Pergamon, New York, 1991, p. 733. |
| [2] | For representative reviews, see:(a) C Zhang, H. Ye, A.F. Moretto, et al., Facile solid-phase construction of indole derivatives based on a traceless,activating sulfonyl linker,Org.Lett.2(2000)89-92;(b) N.N. Wan, Y.L. Yang, W.P. Wang, Z.F. Xie, J.D. Wang, Friedel-Crafts alkylation of indoles with nitroalkenes catalyzed by Cu(Ⅱ)-imine complex, Chin. Chem. Lett. 22(2011) 1155-1158;(c) G.R.Humphrey,J.T.Kuethe,Practical methodologies for the synthesis of indoles, Chem. Rev. 106(2006) 2875-2911;(d) K.Higuchi,T.Kawasaki,Simple indole alkaloids and those with a nonrearranged monoterpenoid unit, Nat. Prod. Rep. 24(2007) 843-868;(e) J. Yang, J. Zhang, T.T. Chen, et al., Sulfamic acid as a cost-effective and recyclable solid acid catalyst for Friedel-Crafts alkylation of indole with α,β-unsaturated carbonyl compound and benzyl alcohol, Chin. Chem. Lett. 22(2011) 1391-1394. |
| [3] | (a) P.S. Prathima, P. Rajesh, J.V. Rao, et al., On water expedient synthesis of 3-indolyl-3-hydroxy oxindole derivatives and their anticancer activity in vitro, Eur. J. Med. Chem. 84(2014) 155-159;(b) J.B. Engel, T. Schoenhals, C. Weidler, et al., Tubulin inhibitor AEZS 112 inhibits the growth of experimental human ovarian and endometrial cancers irrespective of caspase inhibition, Oncol. Rep. 22(2009) 361-367;(c) Y. Wang, X. Tang, Z. Shao, et al., Indole-based alkaloids from deep-sea bacterium Shewanella piezotolerans with antitumor activities, J. Antibiot. 67(2014) 395-399;(d) A. Kamal, Y.V. Srikanth, M.N. Khan, T.B. Shaik, M. Ashraf, Synthesis of 33-diindolyl oxyindoles efficiently catalysed by FeCl3 and their in vitro evaluation for anticancer activity, Bioorg. Med. Chem. Lett. 20(2010) 5229-5231. |
| [4] | (a) S. Peddibhotla, 3-Substituted-3-hydroxy-2-oxindole, an emerging new scaffold for drug discovery with potential anti-cancer and other biological activities, Curr. Bioact. Compd. 5(2009) 20-38;(b) C.V. Galliford, K.A. Scheidt, Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents, Angew. Chem. Int. Ed. 46(2007) 8748-8758;(c) S.P. Ivonin, A.V. Lapandin, A.A. Anishchenko, V.G. Shtamburg, Reaction of arylglyoxals with electron-rich benzenes and π-excessive heterocycles. Facile synthesis of heteroaryl α-acyloins, Syn. Commun. 34(2004) 451-461;(d) P.S. Lai, M.S. Taylor, Preparation of substituted oxazoles by Ritter reactions of α-oxo tosylates, Synthesis 9(2010) 1449-1452. |
| [5] | (a) T. Ueda, M. Inada, I. Okamoto, N. Morita, O. Tamura, Synthesis of maremycins A and D1 via cycloaddition of a nitrone with(E)-3-ethylidene-1-methylindolin-2-one, Org. Lett. 10(2008) 2043-2046;(b) T. Itoh, H. Ishikawa, Y. Hayashi, Asymmetric Aldol reaction of acetaldehyde and isatin derivatives for the total syntheses of ent-convolutamydine E and CPC-1 and a half fragment of madindoline A and B, Org. Lett. 11(2009) 3854-3857;(c) S.N. Lin, Z.Q. Yang, B.H.B. Kwok, M. Koldobskiy, C.M. Crews, Total synthesis of TMC-95A and-B via a new reaction leading to Z-enamides. Some preliminary findings as to SAR, J. Am. Chem. Soc. 126(2004) 6347-6355. |
| [6] | For representative reviews, see:(a) YH. Hui, Y.C. Chen, H.W. Gong, Z.F. Xie, Convenient synthesis of bis(indolyl)alkanes by dithiocarbohydrazone Schiff, base/Zn(ClO4)2·6H2O catalyzed Friedel-Crafts reaction of indoles with imines, Chin. Chem. Lett. 25(2014) 163-165;(b) B.V.S. Reddy, N. Rajeswari, M. Sarangapani, et al., Iodine-catalyzed condensation of isatin with indoles:a facile synthesis of di(indolyl)indolin-2-ones and evaluation of their cytotoxicity, Bioorg. Med. Chem. Lett. 22(2012) 2460-2463;(c) P. Paira, A. Hazra, S. Kumar, et al., Efficient synthesis of 3,3-diheteroaromatic oxindole analogues and their in vitro evaluation for spermicidal potential, Bioorg. Med. Chem. Lett. 19(2009) 4786-4789. |
| [7] | H.M. Li, Y.Q. Wang, L. Deng, Enantioselective Friedel-Crafts reaction of indoles with carbonyl compounds catalyzed by bifunctional cinchona alkaloids, Org. Lett. 8(2006) 4063-4065. |
| [8] | M. Willot, J.C. Chen, J.P. Zhu, Combination of lithium chloride and hexafluoroisopropanol for Friedel-Crafts reactions, Synlett 4(2009) 577-580. |
| [9] | Y.H. Hui, Q. Zhang, J. Jiang, et al., Highly efficient asymmetric. synthesis of 3-indolyl(hydroxy) acetates via Friedel-Crafts alkylation of indoles, J. Org. Chem. 74(2009) 6878-6880. |
| [10] | (a) W.S. Chen, Y.Z. Liu, Z.R. Chen, A highly efficient and practical new allylboronate tartramide for the asymmetric allylboration of achiral aldehydes, Eur. J. Org. Chem.(2005) 1665-1668;(b) W.B. Wu, X.Q. Yuan, J. Hu, et al., Catalytic asymmetric construction of chiral hydropyridazines via conjugate addition of N-monosubstituted hydrazones to enones, Org. Lett. 15(2013) 4524-4527. |
| [11] | (a) J.M. Finefield, R.M. Williams, Synthesis of notoamide J:a potentially pivotal intermediate in the biosynthesis of several prenylated indole alkaloids, J. Org. Chem. 75(2010) 2785-2789;(b) A. Akao, N. Nonoyama, T. Mase, N. Yasuda, Development of large-scale preparations of indole derivatives:evaluation of potential thermal Hazards and studies of reaction kinetics and mechanisms, Org. Process. Res. Dev. 10(2006) 1178-1183;(c) P.Y. Choy, C.P. Lau, F.Y. Kwong, Palladium-catalyzed direct and regioselective C-H bond functionalization/oxidative acetoxylation of indoles, J. Org. Chem. 76(2011) 80-84;(d) J.M. Ontoria, S.D. Marco, I. Conte, et al., The design and enzyme-bound crystal structure of indoline based peptidomimetic inhibitors of hepatitis C virus NS3 protease, J. Med. Chem. 47(2004) 6443-6446. |
| [12] | (a) S. Taliani, M.L. Trincavelli, B. Cosimelli, et al., Modulation of A2B adenosine receptor by 1-benzyl-3-ketoindole derivatives, Eur. J. Med. Chem. 69(2013) 331-337;(b) D.A. James, K. Koya, H. Li, et al., Indole-and indolizine-glyoxylamides displaying cytotoxicity against multidrug resistant cancer cell lines, Bioorg. Med. Chem. Lett. 18(2008) 1784-1787. |
| [13] | (a) Y. Wang, X. Tang, Z. Shao, et al., Indole-based alkaloids from deep-sea bacterium Shewanella piezotolerans with antitumor activities, J. Antibiot. 67(2014) 395-399;(b) M. Yoo, S.U. Choi, K.Y. Choi, et al., Trisindoline synthesis and anticancer activity, Biochem. Biophys. Res. Commun. 376(2008) 96-99. |
| [14] | (a) M.J. Thompson, V. Borsenberger, J.C. Louth, K.E. Judd, B. Chen, Design, synthesis, and structure-activity relationship of indole-3-glyoxylamide libraries possessing highly potent activity in a cell line model of prion disease, J. Med. Chem. 52(2009) 7503-7511;(b) L. Liang, G.D. Rao, H.L. Sun, J.L. Zhang, Aerobic oxidation of primary alcohols catalyzed by copper salts and catalytically active m-hydroxyl-bridged trinuclear copper intermediate, Adv. Synth. Catal. 352(2010) 2371-2377. |
| [15] | (a) J. Hao, S. Taktak, K. Aikawa, et al., Biphenylphosphine-palladium(ii) complexes-catalyzed Friedel-Crafts reaction for the synthesis of α-amino and ahydroxy indolylacetates and diindolylacetates, Synlett(2001) 1443;(b) Y.P. Zhu, M.C. Liu, F.C. Jia, et al., Metal-free sp3 C-H bond dual-(Het)arylation:I2-promoted domino process to construct 2,2-bisindolyl-1-arylethanones, Org. Lett. 14(2012) 3392-3395;(c) H.M. Dong, H.H. Lu, L.Q. Lu, C.B. Chen, W.J. Xiao, Asymmetric Friedel-Crafts alkylations of indoles with ethyl glyoxylate catalyzed by(S)-BINOL-Titanium(IV) complex:direct access to enantiomerically enriched 3-indolyl(hydroxy)acetates, Adv. Synth. Catal. 349(2007) 1597-1603. |
 2016, Vol.27
2016, Vol.27 



