b Post Graduate and Research Department of Chemistry, Jamal Mohamed College, Bharathidasan University, Tiruchirapalli, India;
c Organic Chemistry Division, School of Chemical Sciences, Kasaragod Govt. College, Kannur University, Kannur, India
The basis of invention of new leads for drug designing programs is the synthesis of molecules which are novel yet resemble known biologically active molecules by virtue of the presence of some critical structural features. Certain small heterocyclic molecules act as highly functionalized scaffolds and are known pharmacophores of biologically active and medicinally useful molecules. Hence,the synthesis and investigation of the biological activities of novel heterocyclic compounds is increasingly important in medicinal chemistry [1].
The palladium catalyzed Suzuki-Miyaura cross-coupling reaction between organoboranes and organic halides or pseudohalides has emerged as one of the foremost techniques for the creation of carbon-carbon bonds. The salient features of these reactions are the availability,stability,and non-toxicity of a variety of boronic acids,extensive functional group tolerance and easy access for product isolation. These features have evidently extended its scope in synthetic chemistry and hence this reaction has found widespread use in pharmaceutical industries [2]. Imidazopyrazines belong to an important class of heterocyclic compounds which display a broad spectrum of pharmacological activities which include antioxidant,antidiabetic,and anticancer properties [3]. The diverse applications of imidazopyrazines in the field of luminescence have been extensively reported in literature [4]. The microwave assisted organic synthesis (MAOS) has indisputably become a powerful tool in modern drug discovery laboratories for the construction of versatile chemical entities due often to superior reaction rates,selectivity,and product yields as compared to conventional thermal methodologies [5].
Prompted by these observations and as a continuation of our ongoing research program in the synthesis of biologically active molecules [6],we were interested in synthesizing some C-2 substituted imidazopyrazines which may possess significant pharmacological activities. On continuation of our research on palladium catalyzed cross-coupling reactions [7],it has been planned to apply the Suzuki cross-coupling methodology for the synthesis of a series of 2-substituted-1H-imidazo[4,5-a]pyrazines. In this paper,we report a rapid,facile,and efficient methodology for the synthesis of a series of C-2 substituted imidazopyrazines under microwave irradiation.
2. ExperimentalAll solvents and reagents were obtained from commercial suppliers and used without any further purification. All the reactions were carried out under inert argon atmosphere. Analytical TLC was performed on pre-coated aluminum sheets of silica (60 F254 nm) and visualized by short-wave UV light at l 254. Melting points were determined on an EZ-Melt automated melting point apparatus. 1H NMR spectra were recorded at 400 MHz using an internal deuterium lock. Chemical shifts were measured in δ (ppm). 13C NMR spectra were recorded at 100 MHz using an internal deuterium lock. LC-MS analyses were performed using ESI/APCI,with an ATLANTIS C18 (50 mm × 4.6 mm - 5 μm) column and a flow rate of 1.2 mL/min. Microwave-assisted synthesis was performed in a single mode Biotage Initiator Microwave Synthesizer and temperature was monitored using infrared. The microwave reaction was carried out in a 5mL glass vial and high absorption level was maintained. The conditions
2.1. Procedure for the synthesis of intermediate 2To a solution of 2,3-diamino pyrazine 1,was added CDI in THF,which was then heated at 80 ℃ for 4 h. The reaction completion was monitored by TLC. The mixture was diluted with water and extracted in ethyl acetate,dried in anhydrous sodium sulphate,and distilled under reduced pressure. The crude product was purified by column chromatography to procure the titled compound in 92% yield. Mp: 74-76 ℃; 1H NMR (400 MHz,DMSO-d6): δ 6.84 (br,2H,NH),8.68 (d,2H,J = 8.04 Hz,ArH); 13C NMR (100 MHz,DMSO-d6): δ 135.2,143.7,157.5,LC-MS: Calculated 136.1,Observed 137.1.
2.2. Procedure for the synthesis of intermediate 3To a solution of intermediate 2 in dichloro ethane was added POBr3 at 0 ℃,and the reaction mixture was gradually warmed to ambient temperature. The reaction mass was then heated at 80 ℃ for 6 h. The reaction mixture was poured into crushed ice,basified with NaHCO3,and extracted with DCM and distilled in reduced pressure to render the titled compound in 76% yield. Mp: 83-85 ℃; 1H NMR (400 MHz,DMSO-d6): δ 9.05 (d,2H,J = 7.76 Hz,ArH); 12.6 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 123.9,138.3,146.7; LC-MS: Calculated 198.0,Observed 199.0.
2.3. General procedure for the coupling reactionTo a solution of 2-bromoimidazopyrazine intermediate 3(1 equiv.) in DME-H2O (4:1),were added boronic acid (1.5 equiv.),CsF (3 equiv.),and (A-taphos)2PdCl2 (10 mol%),and the solution was purged with argon and stirred at room temperature for 10min. The reaction solution was then placed in the microwave and heated for 20-30 min at 100 ℃. When TLC and LC-MS showed full consumption of starting materials,the reaction mixture was filtered and dilutedwith ethyl acetate. The ethyl acetate layer was extracted,washed in water,washed in brine,dried over anhydrous sodium sulfate,and distilled in vacuum to get the crude material. The crude product was purified by column chromatography and eluted in varying polarities to obtain the substituted diaryls 4a-n.
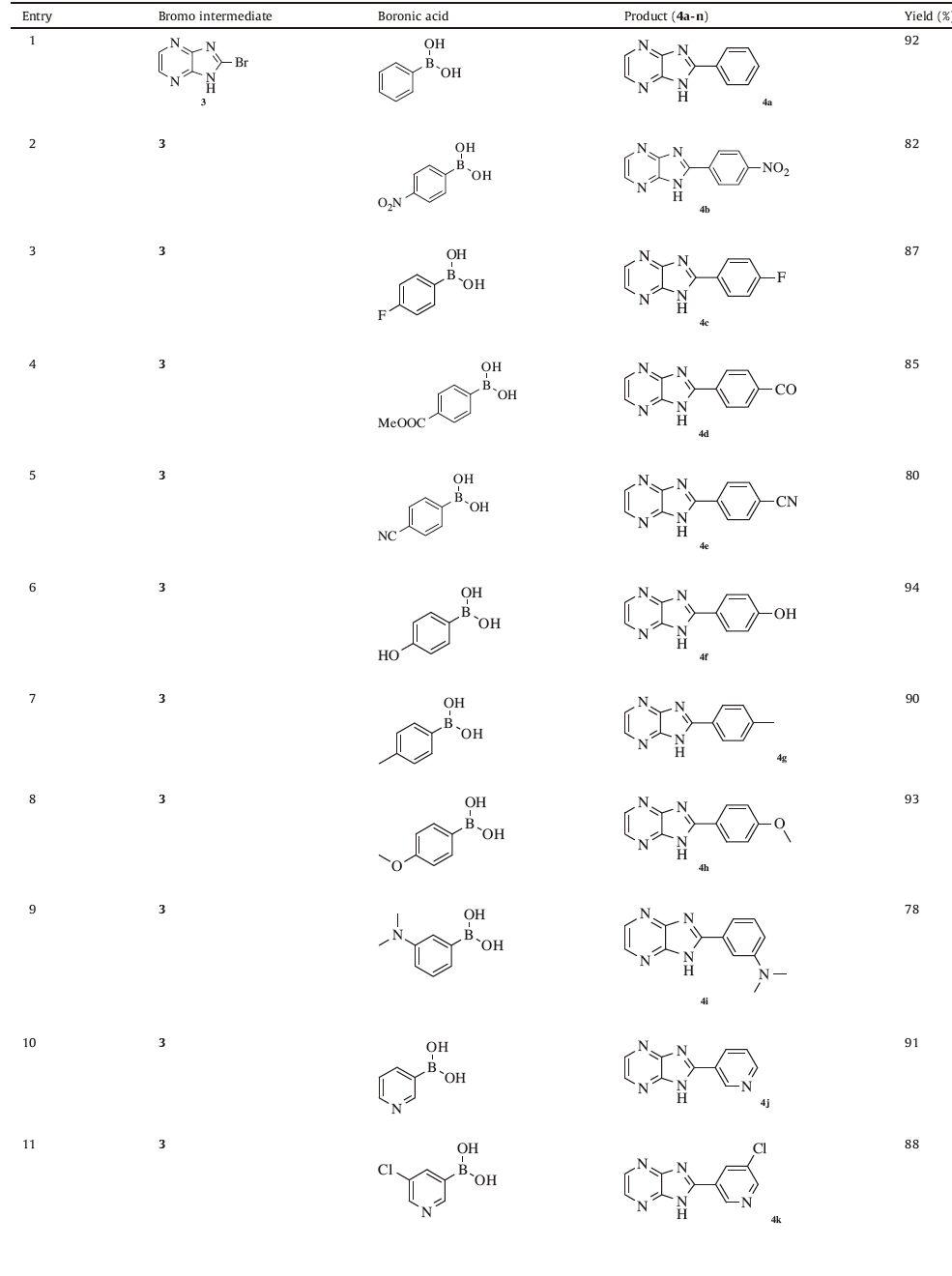
2-Phenyl-1H-imidazo[4,5-a]pyrazine (4a): Mp: 85-87 ℃; 1H NMR (400 MHz,DMSO-d6): δ 7.64-7.86 (m,5H,ArH),8.94 (s,2H,ArH),12.32 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 123.9,129.7,131.2,131.7,133.1,146.6,149.8; LC-MS: Calcd. 196.2,Observed 197.2; Analysis calcd. for C11H8N4: C,67.34,H,4.11,N,28.=,found: C,67.38,H,4.08,N,28.53.
2-(4-Nitrophenyl)-1H-imidazo[4,5-a]pyrazine (4b): Mp: 99- 102 ℃; 1H NMR (400 MHz,DMSO-d6): δ 7.92-7.94 (dd,2H,J1 = 1.96 Hz,J2 = 8.44 Hz,ArH),8.46-8.49 (dd,2H,J1 = 2.08 Hz,J2 = 8.56 Hz,ArH),8.72 (d,2H,J = 7.12 Hz,ArH),12.73 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 123.1 (2 peaks),129.6,138.1,145.8,148.9,149.7; LC-MS: Calcd. 241.2,Observed 242.2; Analysis calcd. for C11H7N5O2: C,54.77,H,2.93,N,29.03,found: C,54.82,H,2.91,N,29.03.
2-(4-Fluorophenyl)-1H-imidazo[4,5-a]pyrazine (4c): Mp: 91- 93 ℃; 1H NMR (400 MHz,DMSO-d6): δ 7.35-7.39 (dd,2H,J1 = 2.16 Hz,J2 = 8.04 Hz,ArH),7.79-7.83 (dd,2H,J1 = 1.76 Hz,J2 = 8.24 Hz,ArH),8.97 (s,2H,ArH),12.44 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 118.3,124.0,129.9,131.5,131.7,146.7,149.9,165.1; LC-MS: Calcd. 214.2,Observed 215.2; Analysis calcd. for C11H7FN4: C,61.68,H,3.29,N,26.16,found: C,61.72,H,3.26,N,26.16.
Methyl 4-(1H-imidazo[4,5-a]pyrazin-2-yl)benzoate (4d): Mp: 106-108 ℃; 1H NMR (400 MHz,DMSO-d6): δ 3.96 (s,3H,OCH3),7.81-7.83 (dd,2H,J1 = 2.44 Hz,J2 = 8.24 Hz,ArH),8.26-8.29 (dd,2H,J1 = 2.64 Hz,J2 = 8.76 Hz,ArH),8.75 (d,2H,J = 6.48 Hz,ArH),12.44 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 53.3,122.9,128.8,132.1 (2 peaks),136.2,146.1,148.8,167.2; LC-MS: Calcd. 254.2,Observed 2=.2; Analysis calcd. for C13H10N4O2: C,61.41,H,3.96,N,22.04,found: C,61.45,H,3.95,N,22.04.
4-(1H-Imidazo[4,5-a]pyrazin-2-yl)benzonitrile (4e): Mp: 96- 98 ℃; 1H NMR (400 MHz,DMSO-d6): δ 7.73-7.80 (m,4H,ArH),8.84 (d,2H,J = 7.28 Hz,ArH),12.52 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 114.4,117.1,122.8,129.7,134.1,136.4,145.9,149.2; LC-MS: Calcd. 221.2,Observed 222.2; Analysis calcd. for C12H7N5: C,65.15,H,3.19,N,31.66,found: C,65.19,H,3.16,N,31.64.
4-(1H-Imidazo[4,5-a]pyrazin-2-yl)phenol (4f): Mp: 90-92 ℃; 1HNMR(400 MHz,DMSO-d6): δ 5.04 (s,1H,OH),7.01-7.04 (dd,2H,J1 = 2.36 Hz,J2 = 8.16 Hz,ArH),7.47-7.49 (dd,2H,J1 = 2.28 Hz,J2 = 8.44 Hz,ArH),8.77 (d,2H,J = 7.44 Hz,ArH),12.34 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 118.0 (2 peaks),122.8 (2 peaks),124.7,130.1,145.8,149.1,159.9; LC-MS: Calcd. 212.2,Observed 213.2; Analysis calcd. for C11H8N4O: C,62.26,H,3.80,N,26.40,found: C,62.31,H,3.78,N,26.38.
2-p-Tolyl-1H-imidazo[4,5-a]pyrazine (4 g): Mp: 88-90 ℃; 1H NMR (400 MHz,DMSO-d6): δ 2.67 (s,3H,CH3),7.32-7.34 (dd,2H,J1 = 2.36 Hz,J2 = 8.28 Hz,ArH),7.65-7.68 (dd,2H,J1 = 1.96 Hz,J2 = 8.36 Hz,ArH),8.92 (d,2H,J = 7.08 Hz,ArH),12.12 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 26.7,123.7,129.4,129.8,131.8,140.7,146.9,149.7; LC-MS: Calcd. 210.2,Observed 211.2; Analysis calcd. for C12H10N4: C,68.56,H,4.79,N,26.65,found: C,68.61,H,4.77,N,26.62.
2-(4-Methoxyphenyl)-1H-imidazo[4,5-a]pyrazine (4 h): Mp: 98-100 ℃; 1H NMR (400 MHz,DMSO-d6): δ 3.81 (s,3H,OCH3),7.09-7.13 (dd,2H,J1 = 2.44 Hz,J2 = 8.36 Hz,ArH),7.51-7.53 (dd,2H,J1 = 1.96 Hz,J2 = 8.48 Hz,ArH),8.84 (d,2H,J = 7.56 Hz,ArH),12.46 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 57.1,116.1 (2 peaks),123.2 (3 peaks),129.8,146.0,148.9,162.1; LC-MS: Calcd. 226.2,Observed 227.2; Analysis calcd. for C12H10N4O: C,63.71,H,4.46,N,24.76,found: C,63.75,H,4.44,N,24.75.
3-(1H-Imidazo[4,5-a]pyrazin-2-yl)-N,N-dimethylbenzenamine (4i): Mp: 109-111 ℃; 1H NMR (400 MHz,DMSO-d6): δ 3.12 (s,6H,CH3),6.85-7.04 (m,4H,ArH),7.45-7.48 (dd,2H,J1 = 2.44 Hz,J2 = 8.36 Hz,ArH),8.96 (s,2H,ArH),12.64 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 42.6,114.5,116.7,119.1,123.8,132.4,133.9,146.6,149.8,152.4; LC-MS: Calcd. 239.3,Observed 240.3; Analysis calcd. for C13H13N5: C,65.25,H,5.48,N,29.27,found: C,65.28,H,5.47,N,29.24.
2-(Pyridine-3-yl)-1H-imidazo[4,5-a]pyrazine (4j): Mp: 86- 88 ℃; 1H NMR (400 MHz,DMSO-d6): δ 7.62 (dd,1H,J1 = 2.36 Hz,J2 = 8.48 Hz,ArH),8.04 (d,1H,J = 6.36 Hz,ArH),8.64-8.69 (m,3H,ArH),12.78 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 122.9 (2 peaks),125.6,134.3,135.6,146.1,148.8 (2 peaks),150.7; LC-MS: Calcd. 197.2,Observed 198.2; Analysis calcd. for C10H7N5: C,60.91,H,3.58,N,35.51,found: C,60.95,H,3.57,N,35.48.
2-(5-Chloropyridin-3-yl)-1H-imidazo[4,5-a]pyrazine (4k): Mp: 107-109 ℃; 1H NMR (400 MHz,DMSO-d6): δ 8.37 (s,1H,ArH),8.75-8.81 (m,4H,ArH),12.83 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 123.0 (2 peaks),130.1,134.9,138.6,145.8,147.9,148.8 (2 peaks); LC-MS: Calcd. 231.6,Observed 232.6 & 234.6; Analysis calcd. for C10H6ClN5: C,51.85,H,2.61,N,30.23,found: C,51.89,H,2.59,N,30.21.
2-(1-Methyl-1H-indol-5-yl)-1H-imidazo[4,5-a]pyrazine (4l): Mp: 116-118 ℃; 1H NMR (400 MHz,DMSO-d6): δ 4.12 (s,3H,NCH3),6.58-6.61 (dd,1H,J1 = 2.24 Hz,J2 = 8.56 Hz,ArH),6.94 (d,1H,J = 7.28 Hz,ArH),7.44-7.48 (m,3H,ArH),8.74,(d,2H,J = 7.04 Hz,ArH) 12.47 (br,1H,NH); 13C NMR (100 MHz,DMSOd6): δ 43.4,104.1,112.8,118.1,120.3,122.9,130.1 (3 peaks),137.1,145.9,148.8; LC-MS: Calcd. 249.3,Observed 250.3; Analysis calcd. for C14H11N5: C,67.46,H,4.45,N,28.10,found: C,67.49,H,4.44,N,28.07.
2-(Furan-2-yl)-1H-imidazo[4,5-a]pyrazine (4m): Mp: 80-82 ℃; 1H NMR (400 MHz,DMSO-d6): δ 6.74-6.78 (m,3H,ArH),8.78 (d,2H,J = 7.16 Hz,ArH),12.68 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 106.4,108.6,123.4,137.1,144.1,145.8,1=.4; LC-MS: Calcd. 186.2,Observed 187.2; Analysis calcd. for C9H6N4O: C,58.06,H,3.25,N,30.09,found: C,58.10,H,3.25,N,30.07.
2-(2-Methylpyridin-4-yl)-1H-imidazo[4,5-a]pyrazine (4n): Mp: 90-92 ℃; 1H NMR (400 MHz,DMSO-d6): δ 2.84 (s,3H,CH3),7.57-7.61 (m,2H,ArH),8.74-8.79 (m,3H,ArH),12.37 (br,1H,NH); 13C NMR (100 MHz,DMSO-d6): δ 26.7,110.9,114.1,123.4,146.5 (3 peaks),148.8,151.1,159.8; LC-MS: Calcd. 211.2,Observed 212.2; Analysis calcd. for C11H9N5: C,62.=,H,4.29,N,33.16,found: C,62.59,H,4.27,N,33.14.
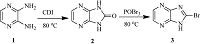
3. Results and discussionThe parent imidazopyrazine core was synthesized by treating the pyrazine 2,3 diamine 1 with CDI in THF at 80 ℃ (Scheme 1) which procured the 2-hydroxyimidazopyrazine intermediate 2. The intermediate thus obtained was further brominated by treating it with POBr3 in dichloroethane at 80 ℃ for 6h to obtain the 2-bromoimidazopyrazine intermediate 3 (Scheme 1). The obtained bromo intermediate was then subjected to the palladium catalyzed Suzuki cross-coupling reactions with diverse boronic acids under microwave irradiation with the aim of synthesizing an array of pharmacologically relevant C-2 substituted imidazopyrazines.
|
Download:
|
| Scheme 1.Synthesis of bromo intermediate 3 | |
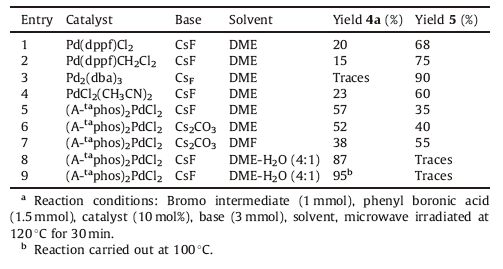
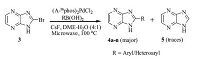
We started our initial screening by coupling the intermediate 3 with phenyl boronic acid since the formation of product could be easily identified by TLC and LC-MS (Scheme 2). A sequence of palladium catalysts in combination with various bases and solvents were investigated at 120 ℃,and the coupling reaction was carried out in a microwave oven for 30min (Table 1). To our disappointment,we could not obtain the required product in acceptable yield in any of the explored conditions and the debrominated product 5 was obtained as the major product.
|
Download:
|
| Scheme 2.Synthesis of various substituted imidazopyrazines. | |
|
|
Table 1 Effect of various catalysts in Suzuki coupling of 3 with phenyl boronic acid.a |
The use of various catalysts like Pd(dppf)Cl2,Pd(dppf)CH2Cl2,PdCl2(CH3CN)2,Pd2(dba)3 etc. were found to be ineffective (Table 1,entries 1-4). Even though we observed the formation of product when (A-taphos)2PdCl2 was used as a catalyst and CsF as base in DME,the reaction could not reach completion even after continuing for 1h (Table 1,entry 5).
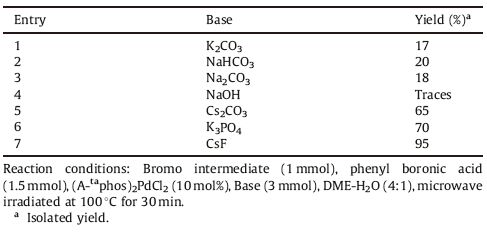
We observed that the nature of base and the solvent system have a determining influence in facilitating the coupling reaction (Table 1,entries 6-8). We presumed that the lack of solubility of all the reagents in the reaction system was the primary reason for the incompleteness of the reaction. The hypothesis proved to be true when we observed that the formation of product was procured in acceptable yield when water was added as a co-solvent to the reaction mixture (Table 1,entry 8). Finally,we obtained the product in excellent yield when DME-H2O (4:1) was used as a solvent system at 100 ℃ (Table 1,entry 9). Among the various bases screened (Table 2),CsF was found to be the best one which rendered the diaryls in exceptional yield with lesser side-products.
|
|
Table 2 Effect of various bases in Suzuki cross-coupling reaction. |
The plausible reason for the debromination could be the attack of metal alkoxides at the Pd(II) oxidative adduct complex followed by hydride migration and reductive elimination [8]. The attack of borate complex at the same Pd(II) oxidative adduct complex leads to the desired coupled product which could be well facilitated by the electronic effect of dimethyl amino group present in (A-taphos)2PdCl2 catalyst,thereby increasing the basicity of the phosphine ligand attached to the palladium center. Similarly,the superiority of CsF to other bases could be rationalized by the fact that CsF generated the most reactive boronate species and facilitated transmetallation quickly which resulted in crosscoupling rather than debromination [9].
With the optimized condition in hands,our next attention was to evaluate the scope of the developed methodology. A series of sterically and electronically diverse boronic acids were coupled with the bromo intermediate 3,and our results are summarized in Table 3. All the boronic acids coupled effectively to render the diaryls in satisfactory to excellent yields. Electron rich boronic acids gave exceptional yields of the coupled product whereas electron deficient boronic acids gave slightly lesser yields (Table 3,entries 2-8). Sterically crowded boronic acids furnished the coupled product in slightly lesser yields even after continuing the reaction for 1h (Table 3,entries 9and 12).
|
|
Table 3 Suzuki coupling of 2-bromoimidazopyrazine with various Boronic acids.a |
A plausible mechanism for the Suzuki cross-coupling reaction has been proposed (Scheme 3). The first step constitutes the oxidative addition of bromo intermediate with palladium to form an organo-palladium species. The subsequent steps involve the formation of a boronate complex which facilitates transmetallation and reductive elimination of the intermediate 3to form the coupled product and thereby completing the catalytic cycle.
4. ConclusionIn summary,we have achieved a rapid,concise and efficient protocol for the synthesis of a series of C-2 substituted imidazopyrazines under microwave irradiation. This method provided an efficient pathway for the synthesis of an assortment of pharmacologically relevant molecules and could be extended for the coupling of other densely functionalized heterocycles in future. The biological screening of the newly synthesized molecules will be conducted in due course and will be communicated shortly.
AcknowledgmentsThe authors are thankful to SAIF,Indian Institute of Technology,Chennai and Indian Institute of Science,Bangalore for providing analytical support. The authors are also grateful to the Department of Industrial Chemistry,Kuvempu University and Head of chemistry department,Govt. College,Kasaragod for providing all the support and facilities for conducting the research work.
| [1] | (a) P.C. Shyma, B. Kalluraya, S.K. Peethambar, S. Telkar, T. Arulmoli, Synthesis, characterization and molecular docking studies of some new 1,3,4-oxadiazolines bearing 6-methylpyridine moiety for antimicrobial property, Eur. J. Med. Chem. 68(2013) 394-404;(b) A.M. Vijesh, A.M. Isloor, S.K. Peethambar, et al., Hantzsch reaction:synthesis and characterization of some new 1,4-dihydropyridine derivatives as potent antimicrobial and antioxidant agents, Eur. J. Med. Chem. 46(2011) 5591-5597. |
| [2] | (a) A.F. Littke, G.C. Fu, Palladium catalyzed coupling reactions of aryl chlorides, Angew. Chem. Int. Ed. 41(2002) 4176-4211;(b) S. Kotha, K. Lahiri, D. Kashinath, Recent applications of the Suzuki-Miyaura cross-coupling reaction in organic synthesis, Tetrahedron 58(2002) 9633-9695;(c) F. Alonso, I.P. Beletskaya, M. Yus, Non-conventional methodologies for transition metal catalyzed carbon-carbon coupling:a critical overview. Part 2:The Suzuki reaction, Tetrahedron 64(2008) 3047-3101;(d) R. Rossi, F. Bellina, M. Lessi, Highly selective palladium-catalyzed Suzuki-Miyaura monocoupling reactions of ethene and arene derivatives bearing two or more electrophilic sites, Tetrahedron 67(2011) 6969-7025. |
| [3] | M.O.C. Galcera, L. Poitout, C. Moinet, et al., Synthesis of substituted imidazopyrazines as ligands for the human somatostatin receptor subtype 5, Bioorg. Med. Chem. Lett. 11(2001) 741-745. |
| [4] | T. Hirano, T. Sekiguchi, D. Hashizume, et al., Colorimetric and fluorometric sensing of the Lewis acidity of a metal ion by metal-ion complexation of imidazo[1,2-a]pyrazin-3(7H)-ones, Tetrahedron 66(2010) 3842-3848. |
| [5] | (a) C.O. Kappe, D. Dallinger, Controlled microwave heating in modern organic synthesis, Mol. Divers. 13(2009) 71-193;(b) E.N. Koini, N. Avlonitis, E.S. Martins-Durate, et al., Divergent synthesis of 2,6-diaryl-substituted 5,7,8-trimethyl-1,4-benzoxazines via microwave-promoted palladium-catalyzed Suzuki-Miyaura cross coupling and biological evaluation, Tetrahedron 68(2012) 10302-10309;(c) C.O. Kappe, Controlled microwave heating in modern organic synthesis, Angew. Chem. Int. Ed. 43(2004) 6250-6284. |
| [6] | (a) A.M. Sajith, K.K. Abdul Khader, N. Joshi, et al., Design, synthesis and structureactivity relationship(SAR) studies of imidazo[4,5-b] pyridine derived purine isosteres and their potential as cytotoxic agents, Eur. J. Med. Chem. 89(2015) 21-31;(b) A. Chandrashekar, B. Eshwarappa, Y.D. Bodke, et al., Synthesis and evaluation of antioxidant properties of novel 1,2,4-triazole-based Schiff base heterocycles, Arch. Pharm. Chem. Life Sci. 346(2013) 922-930;(c) R. Kenchappa, Y.D. Bodke, S.K. Peethambar, S. Telkar, V.K. Bhovi, Synthesis of b-amino carbonyl derivatives of coumarin and benzofuran and evaluation of their biological activity, Med. Chem. Res. 22(2013) 4787-4797. |
| [7] | (a) K.K. Abdul Khader, A.M. Sajith, M.S.A. Padusha, H.P. Nagaswarupa, A. Muralidharan, Cycloalkenyl nonaflates as electrophilic cross-coupling substrates for palladium catalyzed C-N bond forming reactions with enolizable heterocycles under microwave enhanced conditions, New J. Chem. 38(2014) 1294-1305;(b) K.K. Abdul Khader, A.M. Sajith, M.S.A. Padusha, H.P. Nagaswarupa, A. Muralidharan, Regioselective synthesis of C-2 substituted imidazo[4,5-b]pyridines utilizing palladium catalysed C-N bond forming reactions with enolizable heterocycles, Tetrahedron Lett. 55(2014) 1778-1783;(c) M.N. Joy, Y.D. Bodke, K.K. Abdul Khader, A.M. Sajith, A rapid approach for the copper, amine and ligand-free Sonogashira coupling of 4-methyl-7-nonafluorobutylsulfonyloxy coumarins under microwave irradiation, Tetrahedron Lett. 55(2014) 2355-2361;(d) A.M. Sajith, A. Muralidharan, Exploration of copper and amine-free Sonogashira cross coupling reactions of 2-halo-3-alkyl imidazo[4,5-b]pyridines using tetrabutyl ammonium acetate as an activator under microwave enhanced conditions, Tetrahedron Lett. 53(2012) 5206-5210;(e) M.N. Joy, Y.D. Bodke, K.K. Abdul Khader, et al., A rapid and modified approach for C-7 amination and amidation of 4-methyl-7-nonafluorobutylsulfonyloxy coumarins under microwave irradiation, RSC Adv. 4(2014) 19766-19777. |
| [8] | (a) O.O. Navarro, H. Kaur, P. Mahjoor, S.P. Nolan, Cross-coupling and dehalogenation reactions catalyzed by(N-heterocyclic carbene)Pd(allyl)Cl complexes, J. Org. Chem. 69(2004) 3173-3180;(b) J. Moon, S. Lee, Palladium catalyzed-dehalogenation of aryl chlorides and bromides using phosphate ligands, J. Organomet. Chem. 694(2004) 473-477. |
| [9] | (a) A.S. Guram, X. Wang, E.E. Bunel, et al., New catalysts for Suzuki-miyaura coupling reactions of heteroatom-substituted heteroaryl chlorides, J. Org. Chem. 72(2007) 5104-5112;(b) V.F. Slagt, A.H.V. Vries, J.G. Vries, R.M. Kellogg, Practical aspects of carbon-carbon cross-coupling reactions using heteroarenes, Org. Proc. Res. Dev. 14(2010) 30-47;(c) A.M. Sajith, A. Muralidharan, Microwave enhanced Suzuki coupling:a diversity-oriented approach to the synthesis of highly functionalised 3-substituted-2-aryl/heteroaryl imidazo[4,5-b]pyridines, Tetrahedron Lett. 53(2012) 1036-1041. |
 2016, Vol.27
2016, Vol.27