b School of Biosciences and Technology, VIT University, Vellore 632014, India
Staphylococcus aureus,a gram-positive coccal bacterium,infects tissues when the skin or mucosal barriers are breached. Its infections spread through contact with pus from an infected wound,skin-to-skin contact with an infected person by producing hyaluronidase that destroys tissues and contact through the objects used by an infected person. It is estimated that 20% of human population are long term carriers of S. aureus [1]. It remains still as one of the five most common causes of nosocomial infections and is often the cause of post-surgical wound infections. S. aureus,the chief culprit,is also a common source of community acquired infections,and causes illnesses that range from minor skin infections and abscesses to life-threatening diseases such as severe pneumonia,meningitis,joint infections,and heart and blood stream infections [2]. Most current bactericidal compounds inhibit DNA,RNA,cell walls,and protein synthesis [3]. In the case of S. aureus,infection occurs by inhibition of cell wall synthesis by non-lytic cell death. Drug resistant bacterial infections are becoming more prevalent and are a major challenging health issue faced today. This rise of drug-resistance has limited our repertoire of effective antimicrobials that could combat/overcome the problem of drug-resistance.
Inflammation is a complex biological response of vascular tissues to harmful stimuli like pathogens,cells,and irritants [4]. The therapeutic anti-inflammatory effect of nonsteroidal antiinflammatory drugs (NSAIDs) occurs through inhibition of prostaglandin biosynthesis and the selective inhibition of cyclooxygenase. NSAIDs are able to overcome the side-effects of steroid therapy through this mechanism [5, 6]. Further,NSAIDs have many drawbacks such as gastrointestinal toxicity,etc. [6]. In view of this,a new generation of bis(indolyl)methanes as therapeutic agents which nullify these drawbacks are developed. The indole is the most ubiquitous heterocyclic moiety in many biological systems that show pharmacological activity [7, 8]. During the past few years,a large number of natural products containing bis(indolyl) methanes (BIM’s) and bis(indolyl)ethane’s (BIE’s) have been isolated from marine sources. The BIM’s are highly beneficial in promoting estrogen metabolism in women and men [9]. They also exhibit antibacterial [10],cytotoxic [11],insecticidal [12],analgesic [13] and anti-inflammatory activities [13]. Due to this,special interest has been focused on their synthesis [14].
Bis(indolyl)methanes are obtained by reactions of indoles with various aldehydes via azafulvenium salt intermediate in the presence of several Bronsted and Lewis acid catalysts such as LiClO4 [15],CAN [16],ZrOCl2 [17],InCl3 [18],AlPW12O40 [19],ionic liquids [20],trichloro-1,3,5 triazine [21],PFPAT [22],HFIP [23],KHSO4 [24],and molecular iodine [25]. But many of these methods have several drawbacks such as use of expensive reagents,longer reaction times,cumbersome workup,and low product yields. All these procedures involve the use of environmentally toxic solvents and hazardous chemical substrates. Solvent-free organic reactions have become the choice in green chemical organic synthesis [26]. Reports on condensation of indoles with carbonyl compounds under neat conditions are scarce in the literature [27]. Many synthetic chemists have made alternative sustainable and efficient heating procedures to replace the classical thermal heating in synthetic methods. Application of microwave irradiation (MWI) is one such technique employed in effecting organic reactions [28]. MWI assisted organic synthesis requires mild conditions,short reaction times and high product yields. In MWI,the chemical reactions are accelerated because of selective absorption of microwave energy by polar molecules [29, 30].
2. ExperimentalSolvents and reagents were procured from Sigma-Aldrich & Merck and are used as such without further purification. Melting points were determined using a calibrated thermometer by Guna Digital Melting Point apparatus. IR spectra of samples were recorded as potassium bromide pellet on a Bruker Vector 21 FT-IR spectrophotometer. 1H NMR and 13C NMR spectra were recorded as solutions in CDCl3 on a Bruker AMX 500 MHz NMR spectrometer operating at 400 MHz for 1H spectra,and 100 MHz for 13C spectra using tetra methyl silane (TMS) as an internal standard. LCMS mass spectra were recorded on a Jeol SX 102 DA/600 Mass spectrometer. Elemental analysis was performed on a Thermo Finnegan Instrument.
2.1. General procedureA mixture of indole (2 mmol),aldehyde (1 mmol) is placed in 25 mL conical flask and was irradiated by microwave (CATA.4R,Catalyst 300 W,5 min). After completion of the reaction,the reaction mixture is extracted with ethyl acetate and concentrated under reduced pressure. The obtained crude product is purified by column chromatography on silica gel adsorbent using petroleum ether-ethyl acetate mixtures as eluent to obtain known pure 3a,3b,3d,3f,3i,3j,3k,3l,3m,3n and 3c,3e,3g,3h and 3o new compounds.
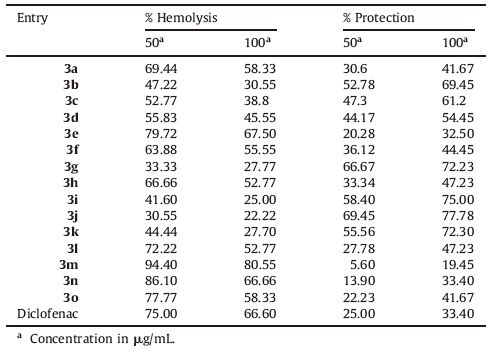
Spectral data for 3,30-((4-(methylthio)phenyl)methylene) bis(1H-indole) (3e): Orange solid; isolated yield: (90%); mp 187-189 ℃; IR: n = 3401 (NH),2928,1684,1497,1217,1085,1014 cm-1; 1H NMR (400 MHz,CDCl3): δ 7.79 (brs,2H,NH) 7.37,6.58 (m,14H,Ar-H),5.82 (s,1H,Ar-CH),2.43 (s,3H,S-CH3); 13C NMR (100 MHz,CDCl3): d 141.2,136.7,130.1,129.3,127.1,126.79,123.7,119.9,53.5,16.1;MS(LCMS):m/z 391 [M + Na]+; Anal. Calcd. for C24H20N2 S: C,78.23,H,5.47,N,7.60,S,8.70. Found: C,78.20,H,5.44,N,7.40,O,8.66.
The compound 3e was also characterized from its powder XRD data (Fig. 1).
|
Download:
|
| Fig. 1.Powder XRD data of compound 3e. | |
Well diffusion method was used to evaluate the antibacterial activity of BIM’s on bacterial species [31]. Bacterial inoculums are prepared by growing a single colony overnight in nutrient broth and adjusting the turbidity to 0.5 McFarland standards. Mueller- Hinton agar (MHA) plates were inoculated with this bacterial suspension and the compounds (1-15) 100 μg/mL were added to a center well with a diameter of 8 mm. These plates were incubated at 37 ℃ for 24 h. The zone of inhibition (ZOI) was measured by subtracting the well diameter from the total inhibition zone diameter. Tetracycline is used as a positive control for bacterial species. All experiments were done in triplicate,and the results are consistent.
2.2.2. In vitro anti-inflammatory assayAlsever’s solution was prepared by dissolving 2% dextrose,0.8% sodium citrate,0.05% citric acid and 0.42% of sodium chloride in distilled water followed by sterilization [32]. Blood was collected from healthy volunteers. The collected blood was mixed in equal volumes with Alsever’s solution. The mixture was centrifuged at 3000 rpm for 10 min and the packed cells were washed three times with isosaline (0.85%,pH 7.2) and 10% (v/v) suspension was made. Test samples with concentrations of 50 mg/mL and 100 mg/mLwere prepared by suspending inDMSO.The assaymixture contained the sample, 1 mL phosphate buffer (pH 7.2,0.1 mol/L),2 mL hyposaline (0.36%),and 0.5 mL human red blood cells suspension. Hydrocortisone sodium was used as the reference drug and 2 mL of distilled water as control. All the assay mixtures were incubated at 37 ℃ for 30 min and centrifuged. The hemoglobin content in the supernatant solution was estimated using a spectrophotometer at 560 nm. The percentage hemolysis was calculated by assuming the hemolysis produced in the presence of distilled water was 100%.
The percentage of hemolysis and stabilization of HRBC membrane are calculated from the following equations: %Hemolysis ¼ ODT/ODC × 100; %Protection ¼ 100- [ODT/ODC] - × 100 where ODT = optical density of the test sample and ODC = optical density of the control.
3. Results and discussion 3.1. ChemistryA facile and clean one pot two component reaction of an aldehyde and indole in 1:2 molar ratio is described for the synthesis of bis(indolyl)methanes under microwave irradiation and catalytic free condition (Table 1). To optimize the solvent requirements,ethanol,toluene,acetonitrile and THF are used and obtained in 70%,62%,58%,43% yields respectively in 15 min under microwave conditions. Under the same neat condition,92% product yield is obtained within 5 min. The scope of application of this method is found by reacting different substituted aromatic and heteroaromatic aldehydes. In all cases,corresponding BIM’s were obtained in 90% yields for heteroaromatic aldehydes while homoaromatic aldehydes afforded poor yields. Among the heteroaromatic aldehydes,six membered derivatives gave higher yields when compared to that of five membered ones.
|
|
Table 1 Synthesis of bis(indolyl)methanes. |
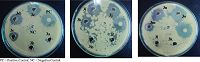
The antibacterial activity of 3a-o is investigated by screening them against the gram positive bacterium S. aureus by well diffusion technique (Fig. 2) with reference to Tetracycline.
|
Download:
|
| Fig. 2.The antibacterial activity of 3a-o by well diffusion analysis | |
The inhibition zone values are summarized in Table 2. The results showed that 3o showed good antibacterial activity whereas 3i,3j showed moderate activity compared to that of the standard drug. All the title compounds are also subjected to in vitro antiinflammatory activity using two different concentrations,50 mg/ mL and 100 mg/mL. Diclofenac is used as the standard. All the tested compounds showed excellent anti-inflammatory activity when compared to their diclofenac except 3m.
|
|
Table 2 Anti-bacterial activity of bis(indolyl)methanes. |
Interestingly,3j,3i,3k,3g exhibited remarkably higher anti-inflammatory activity than that of the standard drug and thus qualifies for further clinical tests to on them so that they can be used as anti-inflammatory agents. The results are summarized in Table 3.
|
|
Table 3 In vitro anti-inflammatory activity of bis(indolyl)methanes. |
Green synthesis of bis(indolyl)methanes by microwave irradiation condition of indole with aldehydes under solvent free conditions is reported. This procedure has short reaction time and affords high product yields. Compound 3o showed good antibacterial activity against S. aureus. The anti-inflammatory activity revealed almost all title compounds except 3m exhibited good anti-inflammatory activity. Compounds 3j,3i,3k and 3g showed much higher anti-inflammatory activity than the standard diclofenac drug and thus qualifying for further clinical evaluation so that they can be used as effective anti-inflammatory agents.
AcknowledgmentWe thank Prof. C.D. Reddy,Department of Chemistry,S.V. University,Tirupati for his helpful discussions and University Grants Commission (UGC),New Delhi,India for providing Senior Research Fellowship (SRF) to Ms. S. Santhisudha under UGC-RGNF (Rajiv Gandhi National Fellowship) scheme and for providing financial assistance through a Major Research Project (F. No. 42- 281/2013 (SR),Dated: 12-03-2013).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.08.012.
| [1] | J. Kluytmans, A. Van Belkum, H. Verbrugh, Nasal carriage of Staphylococcus aureus:epidemiology, underlying mechanisms, and associated risks, Clin. Microbiol. Rev. 10(1997) 505-520. |
| [2] | B. John, Experimental Staph Vaccine Broadly Protective in Animal Studies, National Institute of Health News, 1999. |
| [3] | C. Walsh, Antibiotics:Actions, Origins, Resistance, ASM Press, Washington, DC, 2003. |
| [4] | L. Ferrero-Miliani, O.H. Nielsen, P.S. Andersen, S.E. Girardin, Chronic inflammation:importance of NOD2 and NALP3 in interleukin-1β generation, Clin. Exp. Immunol. 147(2007) 227-235. |
| [5] | A. Kar, Medicinal Chemistry, 2nd ed., New Age International Publishers, New Delhi, 2003, p. 329. |
| [6] | G. Kant, A. Parate, S.C. Chaturvedi, Qsar study of substituted 3,5-di-tert-butyl-4-hydroxy styrene:a series with antiinflammatory activity, Indian J. Pharm. Sci. 67(2005) 116-119. |
| [7] | G.M. Cragg, P.G. Grothaus, D.J. Newman, Impact of natural products on developing new anti-cancer agents, Chem. Rev. 109(2009) 3012-3043. |
| [8] | G.A. Von Cordell, Introduction to Alkaloids:A Biogenetic Approach, Wiley, New York, 1981. |
| [9] | P. Bey, F.N. Bolkenius, N. Seiler, P. Casara, N-(2,3-Butadienyl)-1, 4-butanediamine derivatives:potent irreversible inactivators of mammalian polyamine oxidase, J. Med. Chem. 28(1985) 1-2. |
| [10] | R. Bell, S. Carmeli, N. Sar, Vibrindole A, a metabolite of the marine bacterium, vibrio parahaemolyticus, isolated from the toxic mucus of the boxfish Ostracion cubicus, J. Nat. Prod. 57(1994) 1587-1590. |
| [11] | K. Reddi Mohan Naidu, S.I. Khalivulla, S. Rasheed, et al., Synthesis of bisindolylmethanes and their cytotoxicity properties, Int. J. Mol. Sci. 14(2013) 1843-1853. |
| [12] | M. Lounasmaa, A. Tolvanen, Simple indole alkaloids and those with a nonrearrangedmonoterpenoid unit, Nat. Prod. Rep. 17(2000) 175-191. |
| [13] | K. Sujatha, P.T. Perumal, D. Muralidharan, M. Rajendran, Synthesis, analgesic and anti-inflammatory activities of bis(indolyl)methanes, Indian J. Chem. 48(2009) 267-272. |
| [14] | R.E. Moore, C. Cheuk, X.Q.G. Yang, et al., Hapalindoles, antibacterial and antimycotic alkaloids from the cyanophyte hapalosiphon fontinalis, J. Org. Chem. 52(1987) 1036-1043. |
| [15] | J.S. Yadav, B.V.S. Reddy, C.V.S.R. Murthy, G.M. Kumar, C. Madan, Lithium perchlorate catalyzed reactions of indoles:an expeditious synthesis of bis(indolyl)-methanes, Synthesis(2001) 783-787. |
| [16] | W.L. Deb, P.J. Bhuyan, An efficient and clean synthesis of bis(indolyl)methanes in a protic solvent at room temperature, Tetrahedron Lett. 47(2006) 1441-1443. |
| [17] | R.R. Rahul, D.B. Shinde, Zirconyl(IV) chloride-catalysed reaction of indoles:an expeditious synthesis of bis(indolyl)methanes, Acta Chim. Slov. 53(2006) 210-213. |
| [18] | G. Babu, N. Sridhar, P.T. Perumal, A convenient method of synthesis of bisindolylmethanes:indium trichloride catalyzed reactions of indole with aldehydes and schiff's bases, Synth. Commun. 30(2000) 1609-1614. |
| [19] | H. Firouzabadi, N. Iranpoor, A.A. Jafari, Aluminumdodecatungstophosphate(AlPW12O40), a versatile and a highly water tolerant green Lewis acid catalyzes efficient preparation of indolederivatives, J. Mol. Catal. A Chem. 244(2006) 168-172. |
| [20] | S.J. Ji, J.F. Zhou, D.G. Gu, S.Y. Wang, S.Y. Loh, Efficient synthesis of bis(indolyl)-methanes catalyzed by lewis acids in ionic liquids, Synlett 35(2003) 2077-2079. |
| [21] | G.V.M. Sharma, J.J. Reddy, P.S. Lakshmi, P.R. Krishna, A versatile and practical synthesis of bis(indolyl)methanes/bis(indolyl)glycoconjugates catalyzed by trichloro-1,3, 5-triazine, Tetrahedron Lett. 45(2004) 7729-7732. |
| [22] | S. Khaksar, S.M.J. Ostad, Pentafluorophenylammonium triflate as an efficient, environmentally friendly and novel organocatalyst for synthesis of bis-indolyl methane derivatives, J. Fluorine Chem. 132(2011) 937-939. |
| [23] | A. Kamal, A.A. Qureshi, Syntheses of some substituted di-indolylmethanes in aqueous medium at room temperature, Tetrahedron 19(1963) 513-520. |
| [24] | R. Nagarajan, P.T. Perumal, Potassium hydrogen sulfate-catalyzed reactions of indoles:a mild, expedient synthesis of bis-indolylmethanes, Chem. Lett. 33(2004) 288-289. |
| [25] | S.J. Ji, S.Y. Wang, Y. Zhang, T.P. Loh, Facile synthesis of bis(indolyl)methanes using catalytic amount of iodine at room temperature under solvent-free conditions, Tetrahedron 60(2004) 2051-2055. |
| [26] | A. Loupy, Microwaves in Organic Synthesis, Wiley-VCH, Weinheim, 2006. |
| [27] | M. Chakrabarti, S. Sarkar, Novel clay-mediated, tandem addition-elimination-(Michael) addition reactions of indoles with 3-formylindole:an eco-friendly route to symmetrical and unsymmetrical triindolylmethanes, Tetrahedron Lett. 43(2002) 1351-1353. |
| [28] | G.S. Rashinkar, S.B. Pore, K.B. Mote, R.S. Salunkhe, An efficient synthesis of novel 2-amino-4-aryl-6-ferrocenyl pyrimidine, Indian J. Chem. 48B(2009) 606-610. |
| [29] | B.C. Das, G. Marippan, S. Saha, D. Bhowmik, J. Chiranjib, Anthelmintic and antimicrobial activity of some novel chalcone derivatives, J. Chem. Pharm. Res. 2(2010) 113-120. |
| [30] | S.Y. Wang, S.J. Ji, T.P. Loh, The Michael addition of indole to aα,β-unsaturated ketones catalyzed by iodine at room temperature, Synlett(2003) 2377-2379. |
| [31] | A. Ravaei, Z.H. poor, T.Z. Salehi, et al., Evaluation of antimicrobial activity of three Lactobacillus spp. against antibiotic resistance Salmonella typhimurium, Adv. Stud. Biol. 5(2013) 61-70. |
| [32] | R. Vadivu, K.S. Lakshmi, In vitro and in vivo anti-inflammatory activity of leaves of Symplocos cochinchinensis(Lour) Moore ssp laurina, Bangladesh J. Pharmacol. 3(2008) 121-124. |
| [33] | J.S. Yadav, B.V.S. Reddy, C.V.S.R. Murthy, G.M. Kumar, C. Madan, Lithium perchlorate catalyzed reactions of indoles:an expeditious synthesis of bis(indolyl)-methanes, Synthesis 5(2001) 783-787. |
| [34] | K. Reddi Mohan Naidu, P.S.I. Khalivulla, P. Chenna Rohini Kumar, O. Lasekan, KHSO4-SiO2 catalyzed facile synthesis of bis(indolyl)methanes, Org. Commun. 5(2012) 150-159. |
| [35] | M.A. Zolfigol, P. Salehi, M. Shiri, Z. Tanbakouchian, A new catalytic method for the preparation of bis-indolyl and tris-indolyl methanes in aqueous media, Catal. Commun. 8(2007) 173-178. |
| [36] | S. Handy, N.M. Westbrook, A mild synthesis of bis(indolyl)methanes using a deep eutectic solvent, Tetrahedron Lett. 55(2014) 4969-4971. |
| [37] | S.P.A. Boehringer Mannheim Italia, Bis-Indole derivatives having antimetastatic activity, a process for their preparation and pharmaceutical compositions containing them, Eur. Pat. Appl.(1998) 13, CODEN:EPXXDW; EP887348. |
| [38] | A.K. Mallik, R. Pal, T.K. Mandal, Facile formation of bis(3-indolyl)methylarenes by Iodine-catalyzed reaction of indole with α,α'-bis(arylmethylene)ketones and α-substituted arylmethyleneketones in dry ethanol, Indian J. Chem. 46B(2007) 2056-2059. |
| [39] | R. Martínez, A. Espinosa, A. Tárraga, P. Molina, Bis(indolyl)methane derivatives as highly selective colourimetric and ratiometric fluorescent molecular chemosensors for Cu2+ cations, Tetrahedron 64(2008) 2184-2191. |
 2016, Vol.27
2016, Vol.27