Heat shock protein 90 (Hsp90) is an ATP-dependent 90 kD molecular chaperone ubiquitously expressed in eukaryotes [1]. An array of downstream proteins,which are termed as Hsp90 clients, are dependent on the chaperon function of Hsp90 during the late stage of their synthesis and structure maturation. Under stressed conditions,Hsp90 is also responsible for the structural maintenance and repairing of the clients,to prevent them from aggregation,dysfunction and degradation.
In cancer cells,Hsp90 is 2-10 fold overexpressed and plays critical role in cell survival,growth and tumor progression [2]. A number of well-known oncogenic proteins,for example AKT,Eerb- 2,Hif-1α and telomerase,are now known to be Hsp90 clients. As a consequence,the maintenance of these malignant pathways could be highly dependent on the chaperone function of Hsp90. It was hypothesized that inhibition of Hsp90 in cancer cells could result in the spontaneous depression of its oncogenic clients and thereby disruption of the related signaling pathways,making the molecular chaperone a "one stone two birds" anticancer drug target. The hypothesis,however,was not widely accepted until the seminal study published by Neckers’ team in 1994 [3]. It was demonstrated that benzoquinone ansamycin geldanamycin (1,Fig. 1) was able to bind to the ATP-binding cite of Hsp90 located in its N-terminal domain,and significantly lowered the cellular level of c-Raf,a welldefined oncogenic Hsp90 client. Since then,research attentions devoted to this subject has become intense.
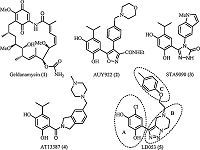
|
Download:
|
| Fig. 1.Selected examples of Hsp90 inhibitors. | |
A large number of Hsp90 inhibitors based on varied scaffolds, mostly targeting the N-terminal ATP-binding pocket of Hsp90, were reported,and nearly twenty of them received clinical studies for treatment of cancer [4, 5]. Among these molecules,resorcinol containing compounds,e.g. AUY-922 (2) [6],STA-9090 (3) [7] and AT13387 (4) [8],made a major class. LD-053(5) is a new resorcinoltype Hsp90 inhibitor identified in our laboratory [9]. It showed notable anticancer activities in several cellular models and was proved to be effective in vivo. We report herein an extended study based on these preliminary results,to demonstrate that the tetrahydro[1, 2, 3]triazolopyrazin central segment presented in LD- 053 actually represents a new scaffold for further discovery of novel Hsp90 inhibitors.
2. Experimental 2.1. Chemicals and instrumentsStarting materials,reagents were purchased from commercial suppliers (Acros,Alfa Aesar) and used without further purification unless otherwise stated. Pyridine (Py.) was dried over solid potassium hydroxide and distilled. Tetrahydrofuran (THF) and toluene were distilled over sodium. Dry dichloromethane (DCM) was distilled over P2O5 before use. Proton nuclear magnetic resonance (1H NMR) spectra and carbon-13 (13C NMR) spectra were recorded on a Varian Mercury-300,Varian Mercury-400, Varian Avance-500 or Varian System-600 spectrometer. 1H spectra was referenced to the residual solvent (δ 7.26 ppm for CDCl3,d 2.04 ppmforCD3COCD3) or tetramethylsilane (δ 0 ppm,CDCl3) as an internal reference. For 13C spectra,chemical shifts are reported relative to the δ 77.0 ppm resonance of CDCl3 or the δ 28.9 ppm resonance of CD3COCD3. Coupling constants are reported in Hz. Infrared (IR) spectra were recorded with Microscope Transmission on a Nicolet 5700 FT-IR. Optical rotations were measured on a Perkin-Elmer 240 using a quartz cellwith 1 mLcapacity and a 10 cm path length at 20 ℃. Mass spectra were recorded on a Thermo Finnigan LTQFTmass spectrometrymanufactured by ThermoFisher Scientific (San Jose,CA,USA). Column chromatography was generally performed using HaiyangZCX. II (200-300 mesh) silica gel. Unless noted otherwise,all compounds isolated by chromatography were sufficiently pure by 1H NMR analysis for use in subsequent reactions.
2.2. Synthesis of compounds 8-13aN-(2-(tert-Butyldimethylsilyloxy)ethyl)-N-(3-(5-isopropyl- 2,4-bis(methoxymethoxy)phenyl)prop-2-ynyl)benzamide (8): 1- Iodo-5-isopropyl-2,4-bis(methoxymethoxy)benzene (6) (2.8g, 7.65 mmol),N-(2-(tert-butyldimethylsilyloxy)ethyl)-N-(prop-2- ynyl)benzamide (7) (2.67 g,8.4 mmol) and CuI (0.29 g, 1.53 mmol) were mixed in Et3N (35 mL) (See Supporting information for preparation of 6 and 7). The mixture was ultrasonically deoxygenated under an argon atmosphere and then PdCl2(PPh3)2 (540 mg,0.77 mmol) was added. The reaction mixture was heated at 60 ℃ for 2 h and filtered. The filtrate was concentrated and the residue was stirred in hexanes (30 mL). The resultingmixture was filtered again. The filtrate was concentrated and the residue purified by chromatography on silica gel (petroleum ether/ethyl acetate,8/1) to give compound 8 (3.34 g,95%) as yellow oil. 1H NMR (400MHz,CDCl3): d 7.66- 7.36 (m,5H),7.22 (s,1H),6.86 (s,1H),5.21 (s,2H),5.20 (s,2H),4.39 (m,2H),4.00-3.58 (m,4H),3.51 (s,3H),3.48 (s,3H),3.32-3.18 (m, 1H),1.22 (d,6H,J = 12.3 Hz),0.90 (s,9H),0.08 (s,6H). 13C NMR (151 MHz,DMSO-d6): d 170.91,157.19,1=.40,136.41,131.15, 130.58,130.00,128.87,127.31,106.10,102.73,95.35,94.59, 87.77,60.63,56.41,56.29,50.26,47.57,41.64,26.37,26.20,23.01, 18.32,-5.02. IR (cm-1): 2228,1614,1256,1219,1082. HRMS (ESI+) calcd. for C31H46O6NSi (M + H)+ =6.3089,found =6.3077.
N-(2-Hydroxyethyl)-N-(3-(5-isopropyl-2,4-bis(methoxymethoxy) phenyl)prop-2-ynyl)benzamide (9): Tetrabutylammonium fluoride (3.49 g,8.4 mmol) was added to a solution of compound 8 (3.33 g,6.0 mmol) in THF (30 mL). The mixture was stirred at 40 ℃ for 6 h and diluted with ethyl acetate (20 mL). The resulting solution was successively washed with water (20 mL × 2) and brine (20 mL). The organic layer was dried over anhydrous Na2SO4 and filtered. The filtrate was concentrated and the residue purified by chromatography on silica gel (petroleum ether/ethyl acetate,5/1) to give alcohol 9 (2.12 g,85%) as light yellow oil. 1H NMR (400 MHz,CDCl3): d 7.73-7.36 (m,5H),7.21 (s, 1H),6.88 (s,1H),5.22 (s,2H),5.20 (s,2H),4.29 (m,2H),3.98 (m,2H), 3.91 (m,2H),3.51 (s,3H),3.48 (s,3H),3.23 (m,1H),1.20 (d,6H, J = 6.9 Hz). 13C NMR (151 MHz,CDCl3): d 173.14,157.23,1=.79, 135.19,131.43,130.54,130.37,128.48,127.34,105.24,101.84, 95.20,94.48,86.41,81.90,61.72,56.39,56.25,49.44,41.92,26.46, 22.71. IR (cm-1): 2228,2101,1643,1502,1262,1081. HRMS (ESI+) calcd. for C25H32O6N (M+H)+442.2224,found 442.2205.
N-(2-Azidoethyl)-N-(3-(5-isopropyl-2,4-bis(methoxymethoxy) phenyl)prop-2-ynyl)benzamide (10): CBr4 (1.08 g,3.2 mmol) was added portion-wide to a solution of compound 9 (1.20 g, 2.7 mmol) and PPh3 (0.85 g,3.2 mmol) in dry DMF (15 mL) at 0 ℃ under argon atmosphere. Upon completion of addition,the mixture was stirred for additional 1 h. NaN3 (0.23 g,3.5 mmol) was added in one portion and the reaction mixture was stirred for 48 h at room temperature before it was diluted with ethyl acetate (30 mL). The resulting mixture was successively washed with water (10 mL × 3) and brine (10 mL). The organic layer was dried over anhydrous Na2SO4 and filtered. The filtrate was concentrated and the residue purified by chromatography on silica gel (petroleum ether/ethyl acetate,8/1) to give compound 10 (0.84 g,66%) as light yellow oil. 1H NMR (300 MHz,CDCl3): d 7.68-7.39 (m,5H),7.22 (s,1H),6.87 (s,1H),5.22 (s,2H),5.20 (s,2H), 4.33 (m,2H),3.87 (m,2H),3.73 (m,2H),3.51 (s,3H),3.49 (s,3H), 3.33-3.16 (m,1H),1.20 (d,J = 6.9,6H). 13C NMR (151 MHz,CDCl3): d 171.60,157.21,1=.71,135.41,131.40,130.64,130.08,128.50, 127.01,105.38,102.00,95.25,94.46,86.01,81.96,56.30,56.22, 49.15,45.27,42.01,26.44,22.68. IR (cm-1): 2960.7,2905.8,2102.2, 1642.5,1501.4,1151.3. HRMS (ESI+) calcd. for C25H31O5N4 (M + H)+467.2289,found 467.2272.
(3-(5-Isopropyl-2,4-bis(methoxymethoxy)phenyl)-6,7-dihydro-[ 1,2,3]triazolo[1,5-a]pyrazin-5(4H)-yl)(phenyl)methanone (11): Compound 10 (520 mg,1.11 mmol) was dissolved in toluene (10 mL) and heated at reflux for 10 h. The reaction mixture was the concentrated and the residue was purified by chromatography on silica gel (petroleum ether/ethyl acetate,2/1) to give 11 (470 mg, 91%) as white solid. 1H NMR (500 MHz,DMSO-d6): d 7.72-7.33 (m, 6H),7.06-6.71 (m,1H),5.24 (s,2H),5.04 (s,2H),4.52 (s,2H),4.34- 3.61 (m,2H),3.=-2.91 (m,9H),1.36 (m,6H). 13C NMR (151 MHz, DMSO-d6): d 170.31,1=.38,152.53,138.96,138.79,135.46, 131.01,130.73,129.20,128.47,127.66,113.94,102.10,95.41, 94.74,56.34,46.34,45.85,44.86,44.13,26.42,23.23.IR (cm-1): 1641.8,1506.4,1150.8,1079.6. HRMS (ESI+) calcd. for C25H31O5N4 (M + H)+467.2289,found 467.2271.
3-(5-Isopropyl-2,4-bis(methoxymethoxy)phenyl)-4,5,6,7-tetrahydro-[ 1,2,3]triazolo[1,5-a]pyrazine (12): Benzamide 11 (4.40 g, 9.43 mmol) was heated at reflux with KOH (1.59 g,28.4 mmol) in a mixture of MeOH/H2O (50 mL,v/v,1/1) for 10 h. After the mixture was cooled to room temperature,water was added and then extracted with ethyl acetate. The organic layers were combined, washed with brine,dried over anhydrous Na2SO4,filtered and evaporated to dryness to give 12 (3.0 g,88%) as white solid. This material is pure enough for the next reaction. 1H NMR (400 MHz, CDCl3): d 7.= (s,1H),6.96 (s,1H),5.23 (s,2H),5.11 (s,2H), 4.42 (t,2H,J = 5.6 Hz),4.14 (s,2H),3.50 (d,3H,J = 5.5 Hz),3.41 (s, 3H),3.35 (t,2H,J = 5.6 Hz),3.33-3.24 (m,1H),1.24 (d,6H, J = 6.9 Hz).13C NMR (151 MHz,CDCl3): d 1=.34,152.74,139.27, 131.70,129.66,128.14,114.40,101.98,95.54,94.66,56.32,56.22, 46.69,42.79,42.52,26.73,22.82. IR (cm-1): 1658.2,1112.7, 1053.0. HRMS (ESI+) calcd. for C18H27O4N4 (M + H)+ 363.2027, found 363.2014.
2-(3-(5-Isopropyl-2,4-bis(methoxymethoxy)phenyl)-6,7-dihydro-[ 1,2,3]triazolo[1,5-a]pyrazin-5(4H)-yl)ethanol (13): K2CO3 (571 mg,4.14 mmol) and 2-bromoethanol (1.04 g,8.29 mmol) was added to a solution of compound 12 (1.00 g,2.76 mmol) in dry acetone (10 mL) in a seal tube. The reaction was kept sealed and stirred at 50 ℃ for 48 h. Water was added and the mixture was extracted with ethyl acetate. The organic layers were combined, washed with brine,dried over anhydrous Na2SO4 and filtered. The filtrate was concentrated and residue purified using silica gel chromatography (petroleum ether/ethyl acetate,1/1) to give compound 13 (1.6 g,95%) as yellow oil. 1H NMR (400 MHz, CDCl3): d 7.54 (s,1H),6.94 (s,1H),5.23 (s,2H),5.10 (s,2H),4.50 (t, 2H,J = 5.6 Hz),3.84 (s,2H),3.70 (s,2H),3.51 (s,3H),3.41 (s,3H), 3.30 (m,1H),3.11 (t,2H,J = 5.6 Hz),2.83-2.75 (m,2H),1.24 (d,6H, J = 6.9 Hz). 13C NMR (151 MHz,CDCl3): d 1=.43,152.66,139.65, 131.84,129.14,128.23,114.22,102.06,95.67,94.63,58.44,58.34, 56.37,56.20,53.44,49.44,45.59,26.74,22.81. IR (cm-1): 3338.6, 2952.0,1510.6,1268.9,1211.0,1144.3,988.1. HRMS (ESI+) calcd. for C20H31O5N4 (M + H)+407.2289,found 407.2274.
2-(3-(5-Isopropyl-2,4-bis(methoxymethoxy)phenyl)-6,7-dihydro-[ 1,2,3]triazolo[1,5-a]pyrazin-5(4H)-yl)ethyl methanesulfonate (13a): Compound 13 (200 mg,0.49 mmol) and Et3N (60 mg,0.59 mmol) were dissolved in dry dichloromethane (2.5 mL). Methanesulfonyl chloride (62.4 mg,0.54 mmol) was added dropwise with stirring at 0 ℃. The mixture was kept stirred for 3 h and quenched by addition of water. The mixture was extracted with dichloromethane (10 mL × 3). The organic layers were combined,washed with brine (20 mL),dried over anhydrous Na2SO4. After filtration,the filtrate was concentrated under reduced pressure to yield intermediate 13a. This crude material was used directly in the next step.
2.3. General method for synthesis of compound 15Substituted phenol (1.0 equiv) was dissolved in dry DMF. NaH (3.0 equiv) was added portion-wise at 0 ℃ under stirring. Upon completion of addition,stirring was continued for another 0.5 h. A solution of crude 13a (1 equiv) in dry DMF (1 mL) was injected to the reaction mixture through a syringe. The ice bath removed and the mixture was stirred for 8 h at room temperature before water was added dropwise at 0 ℃ to quench the reaction. The mixture was then extracted with ethyl acetate. The organic layers were combined,washed with brine and then concentrated. The residue was purified by chromatography on silica gel (petroleum ether/ ethyl acetate,1/1) to give 14. 14 was dissolved in HCl-methanol, and stirred at room temperature for 12 h. Then the reaction mixture was evaporated under reduced pressure at 35 ℃ to give the crude product. The crude product was purified by chromatography on silica gel (petroleum ether/ethyl acetate,1/1) to give 15 as white solid.
Characterization data from compound 15-1 to 15-9 can be found in Supporting information.
3. Results and discussionThe validated druggability of Hsp90 has provoked numerous attempts aiming at novel Hsp90 inhibitors,leading to the discovery of stockpiles of small molecules targeting Hsp90. Despite that the structures of these agents ranging from semisynthetic natural product derivatives to target based rational-designed compounds, chemical space within this scope still remains under-explored. Novel structures possessing elevated potency,reduced off-target toxicity and more favorable pharmacokinetic profiles are still of considerable interest. In this context,randomly discovered LD053 positioned us at a good start point for further investigations,and hence an extended series of compounds were designed out of this prototype molecule to tackle a general view of structure activity relationships.
The molecule of LD053 (5,Fig. 1) virtually comprises three sections. We intended to retain the framework except that a more commonly adopted iso-propyl was used in place of the chlorine in section "A". The major variations,on the other hand,were made to section "C",into which a series of mono-substituted phenoxyls were to be introduced with the substituent varying from simple alkyls,halogens to more polar sulfonylsand the site of substitution including othro-,meta- and para-. The designed molecules were synthesized as depicted in Scheme 1. Thus,Sonogashira coupling between 1-iodo-5- isopropyl-2,4-bis(methoxymethoxy)benzene 6 and N-propargyl benzamide 7 to give compound 8 [10]. The product was desilylated (9) and brominated. Upon treatment of the bromo intermediate with NaN3 in DMF,the resultant azide 10 was heated at reflux in toluene to effect the intramolecular [3 + 2] cycloaddition,giving tetrahydro-[1, 2, 3]-triazolopyrazin adduct 11 in 91% yield. Removal of the N-benzoyl by using regular basic hydrolysis condition led to the isolation of 12,which was heated with 2-bromoethanol and K2CO3 in acetone at 50 ℃ in a seal tube to give compound 12. Alcohol 12 was mesylated and coupled with the selected phenols in the presence of sodium hydride in DMF to deliver compound 14-1a-14-9c. These precursors were then stirred in methanolic hydrochloride to remove the MOM protection,giving the final products (15-1a- 15-9c) mostly in forms of precipitated solids. Structures of these compounds were summarized in Table 1.
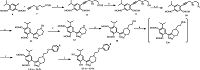
|
Download:
|
| Scheme 1.The synthesis of LD053 analogues. Reagent and conditions: (a) PdCl2(PPh3)2 (0.1 equiv.), CuI (0.2 equiv), Et3N, 60 ℃, 95%; (b) TBAF, THF, 40 ℃, 85%; (c) CBr4, PPh3, DMF, 0 ℃; (d) NaN3, DMF, r.t., 66% for 2 steps; (e) toluene, reflux, 91%; (f) KOH (3 equiv.), MeOH/H2O (v/v 1/1), 88%; (g) BrCH2CH2OH, K2CO3, dry acetone, 50 ℃ in seal tube, 95%; (h) MsCl, Et3N, 0 ℃; (i) Selected phenols, NaH, DMF, 0 ℃ to r.t.; (j) HCl-MeOH (1 M), r.t., 65%-88% for 3 steps. | |
|
|
Table 1 Cancer cell growth inhibitory effects of compound 15 |
Compounds synthesized as described above were submitted to MTT assays [9] in five different cancer cell lines to determine their in vitro effects on cell growth (Table 1),and seven were found active (entries 14,15,18,20,23,24 and 27) with the average IC50s lower than 10 μmol/L. It is noteworthy that simple aliphatic (chlorine as well) substituted compounds in this series generally exhibited low activities (entries 1-12,Table 1). This is also the case for the molecules carrying othro-substitution in section C (entries 13,16, 19,22 and 25),indicating substitution at this position is detrimental to the compounds’ anticancer activity. For the active compounds,on the other hand,a polar substituent,either at metaor para-position,seemed necessary; yet more interesting is that both electron donating and withdrawing groups contributed positively. These selected compounds were further subjected to a polarized fluorescent assay using the known protocol [9] to determine their affinities toward hsp90. As it is showed in Table 2, all compounds appeared highly potent upon binding to purified hsp90,indicating that the anti-cancer activity of these molecules is associated with their capability of hsp90 inhibition. It is unfortunate that the high target affinity of our compounds was not fully reflected in the cellular level examinations,but the results still evidenced that the 4,5,6,7-tetrahydro-[1, 2, 3]triazolo[1,5-a]pyrazine substructure,or section B of the target molecules,may represent a new scaffold for the discovery of novel hsp90 inhibitors.
|
|
Table 2 Hsp90 affinity of the selected compounds. |
In summary,a library of analogues was designed and synthesized based on our randomly discovered prototype hsp90 inhibitor,LD053. In vitro anticancer activities were tested in a panel of cellular tumor models,and seven of the compounds were found active (IC50 2-10 μmol/L). The selected active compounds also exhibited notable binding affinities toward purified hsp90 (IC50 60-100 nmol/L). These results,in combination to our earlier findings,helped the recognition of the 4,5,6,7-tetrahydro- [1, 2, 3]triazolo[1,5-a]pyrazine motif as a new scaffold for hsp90 inhibitors. With the aid of the preliminary structure activity relationships illustrated in this study,further optimization of the molecule is ongoing.
AcknowledgmentThis project is financially supported by the National Natural Science Foundation of China (No. 21272279). Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.09.024.
| [1] | P. Csermely, T. Schnaider, C. Soti, Z. Prohászka, The 90-kDa molecular chaperone family:structure, function and clinical applications, Pharmacol. Ther. 79(1998) 129-168. |
| [2] | (a) R. Garcia-Carbonero, A. Carnero, L. Paz-Ares, Inhibition of HSP90 molecular chaperones:moving into the clinic, Lancet Oncol. 14(2013) e358-e369;(b) H. Yamaki, M. Nakajima, K.W. Shimotohno, N. Tanaka, Molecular basis for the actions of Hsp90 inhibitors and cancer therapy, J. Antibiotics 64(2011) 635-644. |
| [3] | (a) L. Whitesell, E.G. Mimnaugh, B. De Costa, C.E. Myers, L.M. Neckers, Inhibition of heat shock protein HSP90-pp6Ov-src heteroprotein complex formation by benzoquinone ansamycins:essential role for stress proteins in oncogenic transformation, Proc. Natl. Acad. Sci. U.S.A. 91(1994) 8324-8328;(b) T.W. Schulte, W.G. An, L.M. Neckers, Geldanamycin-induced destabilization of Raf-1 involves the proteasome, Biochem. Biophys. Res. Commun. 239(1997) 655-659. |
| [4] | (a) K. Jhaveri, T. Taldone, S. Modi, G. Chiosis, Advances in the clinical development of heat shock protein 90(Hsp90) inhibitors in cancers, Biochim. Biophys. Acta 1823(2012) 742-755;(b) F. Zagouri, T.N. Sergentanis, D. Chrysikos, et al., Hsp90 inhibitors in breast cancer:a systematic review, The Breast 22(2013) 569-578;(c) R. Bhat, S.R. Tummalapalli, D.P. Rotella, Progress in the discovery and development of heat shock protein 90(Hsp90) inhibitors, J. Med. Chem. 57(2014) 8718-8728;(d) L. Neckers, J.B. Trepel, Stressing the development of small molecules targeting HSP90, Clin. Cancer Res. 20(2014) 275-277;(e) K. Jhaveri, S.O. Ochiana, M.P.S. Dunphy, et al., Heat shock protein 90 inhibitors in the treatment of cancer:current status and future directions, Expert Opin. Investig. Drugs 23(2014) 611-628. |
| [5] | (a) J.-M. Jia, F. Liu, X.-L. Xu, et al., Synthesis and evaluation of a novel class Hsp90 inhibitors containing 1-phenylpiperazine scaffold, Bioorg. Med. Chem. Lett. 24(2014) 1557-1561;(b) J. Ren, J. Li, Y. Wang, et al., Identification of a new series of potent diphenol HSP90 inhibitors by fragment merging and structure-based optimization, Bioorg. Med. Chem. Lett. 24(2014) 2525-2529;(c) M. Taddei, S. Ferrini, L. Giannotti, et al., Synthesis and evaluation of new Hsp90 inhibitors based on a 1,4,5-trisubstituted 1,2,3-triazole scaffold, J. Med. Chem. 57(2014) 2258-2274. |
| [6] | P.A. Brough, W. Aherne, X. Barril, et al., 4,5-Diarylisoxazole hsp90 chaperone inhibitors:potential therapeutic agents for the treatment of cancer, J. Med. Chem. 51(2008) 196-218. |
| [7] | D.A. Proia, R.C. Bates, Ganetespib and hsp90:translating preclinical hypotheses into clinical promise, Cancer Res. 74(2014) 1294-1300. |
| [8] | A.J. Woodhead, H. Angove, M.G. Carr, et al., Discovery of(2,4-dihydroxy-5-isopropylphenyl)-[5-(4-methylpiperazin-1-ylmethyl)-1,3-dihydroisoindol-2-yl]methanone(AT13387), a novel inhibitor of the molecular chaperone Hsp90 by fragment based drug design, J. Med. Chem. 53(2010) 5956-5969. |
| [9] | C. Lu, D. Liu, J. Jin, et al., Inhibition of gastric tumor growth by a novel Hsp90 inhibitor, Biochem. Pharmacol. 85(2013) 1246-1256. |
| [10] | R. Severin, J. Reimer, S. Doye, One-pot procedure for the synthesis of unsymmetrical diarylalkynes, J. Org. Chem. 75(2010) 3518-3521. |
 2016, Vol.27
2016, Vol.27 




