b Shanghai Collaborative Innovation Center for Biomanufacturing Technology, Shanghai 200237, China
Neonicotinoids are the most important class of synthetic insecticides in crop protection in the past decades. They represented 28.5% of global market for insecticides and 80% of seed treatment sales in 2011 [1]. Applied into the soil or to the seed,neonicotinoids are taken up via the roots,are distributed in the plant and give consistent and long-lasting control of sucking insect pests such as aphids,whiteflies,planthoppers,some micro lepidoptera and a number of coleopteran pest species [2]. However,due to similar modes of action of neonicotinoids,the problems of resistance and cross-resistance have become an increasing concern [3, 4, 5]. Previous studies reported that some species showed more than 100-fold resistance to imidacloprid (1,Fig. 1) [3, 5]. Therefore,in order to combat current resistance and prevent further development of resistant strains,there is an urgent need to develop novel classes of neonicotinoids [6, 7].

|
Download:
|
| Fig. 1.Imidacloprid and representative neonicotinoids discovered in our group | |
Bicyclic pyridone scaffolds are ubiquitous in a lot of biologically active compounds and natural products [8, 9, 10]. Some of them possess a broad range of biological properties,including insecticidal [11],antibacterial [8, 12],anticancer [13] and anti-HIV activities [14, 15]. Hence,the synthesis of tetrahydroimidazo[1, 2-a]pyridin-5(1H)-one is a valuable strategy for discovering new bioactive compounds [11]. Reactions of 4-(substituted benzylidene)- 2-phenyloxazole-5-ones with heterocyclic ketene aminals via aza-ene reaction have been applied for the creation of diverse heterocycles [16]. However,such aza-ene reaction with βnitroenamines is scarcely reported.
For neonicotinoids,the coplanar system between the electronegative pharmacophore (=CNO2 or =NNO2) and guanidine- amidine moiety extends the conjugation and facilitates negative charge (δ-) flow toward the tip,enhances binding affinity with novel nicotinic acetylcholine receptors (nAChRs),and has been proven as an important pharmacophore [17]. In previous studies,2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine (β-nitroenamines 6-Cl-PMNI) was used intensively as starting materials due to it containing two reactive nucleophilic sites as shown in Fig. 2. (A) α-Carbon atom of the nitromethylene group could be attack by electrophilic agents; (B) β-secondary amine group in imidazolidine also has good reactivity. The obtained compounds with the electronegative pharmacophore (=CNO2),such as Paichongding,IPP152201,and cycloxaprid,exhibited excellent insecticidal activity [18, 19, 20, 21, 22],which encouraged us to further explore structural modification and diversification of these neonicotinoids derivatives.

|
Download:
|
| Fig. 2.Design strategy of tetrahydroimidazo[1, 2-a]pyridin-5(1H)-one derivatives. | |
Enlightened by all of intriguing reasons above,we examined the reaction behavior of b-nitroenamine 6-Cl-PMNI with 4-(substituted benzylidene)-2-phenyloxazole-5-ones. Herein,a series of tetrahydroimidazo[1, 2-a]pyridin-5(1H)-one derivatives were designed and synthesized. Their insecticidal activities against cowpea aphids (Aphis craccivora) and brown planthopper (Nilaparvata lugens) were evaluated.
2. Experimental 2.1. ChemistryReagents and solvents were obtained from commercial sources and used without further purification. Melting points (mp) were determined on a Buüchi Melting Point B540 and were uncorrected. Nuclear magnetic resonance spectra were recorded on a Bruker AM-400 instruments in DMSO-d6 solvent (1H NMR at 400 MHz,13C NMR at 100 MHz,and 19F NMR at 376 MHz). Chemical shifts are given in parts per million (ppm) with tetramethylsilane as an internal standard. High-resolution mass spectra (HRMS) were determined on a MicroMass GCT CA 0= instrument. All MS experiments were performed using electrospray ionization (ESI) in positive ion mode. Reaction progress was determined by thin layer chromatography (TLC) analysis on precoated plates (silica gel 60 F254),and spots were visualized with ultraviolet (UV) light.
Synthesis of benzoyl glycine 8: Glycine (0.1 mol) was dissolved in 75 mL of 10% sodium hydroxide solution. To this,benzoyl chloride (7,0.115 mol) in five portions was added with stirring until benzoyl chloride completely reacted. The solution was transferred to beaker and rinsed the conical flask with a little water. A few gram of crushed ice was added in the solution. Then concentrated hydrochloric acid was added slowly with stirring until the mixture was acidic. The crystalline precipitate of benzoyl glycine was filtered,washed with carbon tetrachloride and then with cold water. The solid product was collected,dried and recrystallized in boiling water. Yield: 90%; mp: 85.1-85.6 ℃. General procedure for preparation of 9a-9r: A mixture of aldehyde (0.1 mol),benzoyl glycine (8,0.1 mol),acetic anhydride (0.3 mol) and anhydrous potassium acetate (0.1 mol) was heated to 90 ℃ till the mixture liquefied. Then the reaction mixture was stirred on an oil bath and monitored by TLC. After 3 h,to this 10 mL of ethanol was added slowly and allowed the mixture to stand overnight at room temperature. The crystalline product was separated by filtration,washed with 5 mL of ice-cold alcohol and then finally washed with 5 mL of boiling water and recrystallized using suitable solvent.
General procedure for preparation of 10a-10r: A solution of 4-(substituted benzylidene)-2-phenyloxazole-5-ones (9a-9r,8.0 mmol),6-Cl-PMNI (10.0 mmol),and hydrobromic acid (10 mmol) in 50 mL acetonitrile at refluxing temperature was stirred. The reaction progress was monitored by TLC. After completion of the reaction,the organic solvent was extracted thoroughly with CH2Cl2 (30 mL × 3),washed with water,and dried with anhydrous Na2SO4. The solvent was removed under reduced pressure. The residue was purified by flash chromatography eluting with dichloromethane/acetone (4:1,v/v) to afford target products 10a-10r. Physical and spectroscopic characterization data of compounds 10a-10r were given in Supporting information.
2.2. Biological assayBioassays were performed on representative test organisms grown in the laboratory. The bioassay was repeated at (25 ± 1) ℃ according to statistical requirements. All compounds were dissolved in N,N-dimethylformamide (AP,Shanghai Chemical Reagent Co.,Ltd.,Shanghai,China) and diluted with distilled water containing Triton X-100 (0.1 mg L-1 ) to obtain a series of concentrations of 500.0,100.0 mg L-1 and others for bioassays. For comparative purposes,avermectins and cycloxaprid as control was tested under the same conditions.
Insecticidal test for cowpea aphids (A. craccivora): The activities of insecticidal compounds against cowpea aphids were tested by leaf-dip method. The leaves of the horsebean plant with 40-60 apterous adults were dipped in diluted solutions of the chemicals containing Triton X-100 (0.1 mg L-1) for 5 s and the excess solution was sucked out with filter paper,and the burgeons were placed in the conditioned room ((25 ± 1) ℃,50% RH). Water containing Triton X-100 (0.1 mg L-1) was used as a control. The mortality rates were evaluated 48 h after treatment. Each treatment had three repetitions and the data were adjusted and subjected to probit analysis as before.
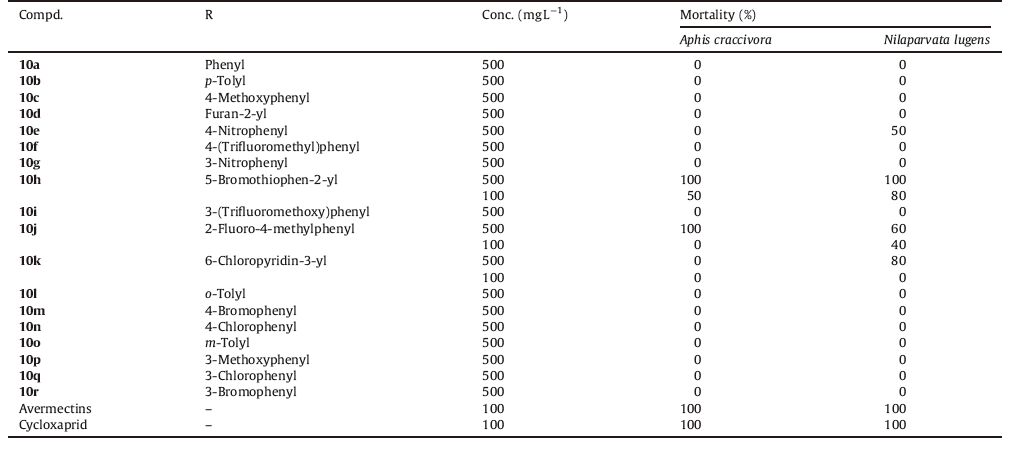
Insecticidal test for brown planthopper (N. lugens): The insecticidal activity against brown planthopper was tested by foliar application. Rice seedlings were placed on moistened pieces of filter paper in Petri dishes. The dishes were infested with third instar larvae and then sprayed with the compound solutions (2.5 mL) using a Potter spray tower (pressure,5 lb (in2)-1; settlement,4.35 mg (cm2)-1). Samples were placed in the conditioned room. The mortality rates were evaluated 48 h after treatment. Each treatment had three repetitions,and the data were adjusted and subject to probit analysis as before. The results of bioassay are depicted in Table 1.
|
|
Table 1 Insecticidal activity of compounds 10a-10r against Aphis craccivora and Nilaparvata lugens. |
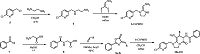
The general strategy for preparing title compounds 10a-10r is outlined in Scheme 1. The commercially available benzoyl chloride 7 was selected as the starting material,which was reacted with glycine in presence of NaOH to afford the benzoyl glycine 8 by the general condensation reaction with a 90% yield. With the key intermediated 8 in hand,conversion of 8 to 9 was then carried out with aromatic aldehyde in the acetic anhydride using NaOAc as base. The compound 9a-9r were obtained in moderate to high yields. From precursor 2-chloro-5-chloromethylpridine 5,the desired β-nitroenamines 6-Cl-PMNI was readily synthesized according to the conventional method [23].
|
Download:
|
| Scheme 1.General synthetic route for title compounds 10. | |
Finally,target compounds 10a-10r,which contain various substituents at the N-7 position (R) of tetrahydroimidazo[1,2- a]pyridin-5(1H)-one,were obtained through an aciδ-catalyzed aza-ene reaction between 9a-9r and 6-Cl-PMNI. Then the reaction condition was investigated to optimize the yield. The examination of catalyst,solvent and temperature led to the following informative observations: (i) hydrobromic acid was the optimal catalyst; (ii) acetonitrile was the suitable solvent; (iii) the product yield was highest at refluxing temperature. It was observed that electron donating or electron-withdrawing groups on the substituents (R) had significant effect on the yield of target product,which indicated that the steric effect and electronic effect affected the reactions. For example,with the OMe group at 4- position,compound 10c was 25% yield. Otherwise,compound 10e with NO2 group at 4-position was 65% yield.
3.2. Insecticidal activityTo evaluate the overall insecticidal activities of tetrahydroimidazo[1, 2-a]pyridin-5(1H)-one derivatives,N. lugens and A. craccivora were selected as the target pests. The testing results against two insects were summarized in Table 1. The preliminary bioassays indicated that 10e,10h,10j,and 10k showed favorable activity,and had 50%-100% mortality against brown planthopper at 500 mg L-1 . Especially,compound 10h exhibited 80% insecticidal activity against N. lugens at 100 mg L-1. Among monosubstituted derivatives,all title compounds was inactive against target pests except for compound 10e. By contrast,multisubstituted compounds 10h and 10j exhibited relatively higher mortality. All these results suggested that activities varied significantly depending on the types and patterns of the substituents. In comparison with avermectins and cycloxaprid,the activities of the target compounds were not high enough,which might be caused by their low water solubility and the large molecular size.
4. ConclusionIn summary,a series of tetrahydroimidazo[1,2-a]pyridin- 5(1H)-one derivatives were designed and synthesized. Their structures were characterized by 1H NMR,13C NMR,19F NMR and HRMS. The bioassays showed that some of the compounds exhibited good activity against brown planthopper (N. lugens) and cowpea aphids (A. craccivora); insecticidal activity was significantly influenced by the substituents. The current results of novel compounds provide important information and further studies on the structural optimizations are in progress.
AcknowledgmentThis work was financial supported by National High Technology Research Development Program of China (863 Program,No. 2011AA10A207),National Natural Science Foundation of China (Nos. 21472046,21372079),Shanghai Pujiang Program (No. 14PJD012) and the Fundamental Research Funds for the Central Universities (No. 222201414015). This work was also partly supported by Australia DC Foundation.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.10. 002.
| [1] | R. Nauen, U. Ebbinghaus-Kintscher, A. Elbert, P. Jeschke, K. Tietjen, Acetylcholine receptors as sites for developing neonicotinoid insecticides, in:I. Ishaaya(Ed.), Biochemical Sites of Insecticide Action and Resistance, Springer Berlin Heidelberg, Berlin, 2001, pp. 77-105. |
| [2] | P. Jeschke, R. Nauen, M.E. Beck, Nicotinic acetylcholine receptor agonists:a milestone for modern crop protection, Angew. Chem. Int. Ed. 52(2013) 9464-9485. |
| [3] | R. Nauen, I. Denholm, Resistance of insect pests to neonicotinoid insecticides:current status and future prospects, Arch. Insect Biochem. Physiol. 58(2005) 200-215. |
| [4] | N. Rauch, R. Nauen, Identification of biochemical markers linked to neonicotinoid cross resistance in Bemisia tabaci(Hemiptera:Aleyrodidae), Arch. Insect Biochem. Physiol. 54(2003) 165-176. |
| [5] | D. Mota-Sanchez, R.M. Hollingworth, E.J. Grafius, D.D. Moyer, Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata(Say)(Coleoptera:Chrysomelidae), Pest Manag. Sci. 62(2006) 30-37. |
| [6] | Y.F. Fan, W.W. Zhang, X.S. Shao, et al., Facile three-component synthesis and insecticidal evaluation of hexahydroimidazo[1,2-a]pyridine derivatives, Chin. Chem. Lett. 26(2015) 1-5. |
| [7] | N.Y. Chen, L.P. Ren, M.M. Zou, et al., Design, synthesis and insecticidal activity of spiro heterocycle containing neonicotinoid analogs, Chin. Chem. Lett. 25(2014) 197-200. |
| [8] | K.L. Eley, L.M. Halo, Z.S. Song, et al., Biosynthesis of the 2-pyridone tenellin in the insect pathogenic fungus Beauveria bassiana, ChemBioChem 8(2007) 289-297. |
| [9] | K. Schmidt, W. Günther, S. Stoyanova, et al., Militarinone A, a neurotrophic pyridone alkaloid from Paecilomyces militaris, Org. Lett. 4(2002) 197-199. |
| [10] | K. Schmidt, U. Riese, Z.Z. Li, M. Hamburger, Novel tetramic acids and pyridone alkaloids, militarinones B, C, and D, from the insect pathogenic fungus Paecilomyces militaris, J. Nat. Prod. 66(2003) 378-383. |
| [11] | L.P. Ren, Y.P. Lou, N.Y. Chen, et al., Synthesis and insecticidal activities of tetrahydroimidazo[1,2-a]pyridinones:further exploration on cis-neonicotinoids, Synth. Commun. 44(2014) 858-867. |
| [12] | J. Alder, J. Clement, J. Meulbroek, N. Shipkowitz, et al., Efficacies of ABT-719 and related 2-pyridones, members of a new class of antibacterial agents, against experimental bacterial infections, Antimicrob. Agents Chemother. 39(1995) 971-975. |
| [13] | L.N. Yin, Q.Z. Hu, R.W. Hartmann, Tetrahydropyrroloquinolinone type dual inhibitors of aromatase/aldosterone synthase as a novel strategy for breast cancer patients with elevated cardiovascular risks, J. Med. Chem. 56(2013) 460-470. |
| [14] | T. Kawasuji, B.A. Johns, H. Yoshida, et al., Carbamoyl pyridone HIV-1 integrase inhibitors. 2. Bi-and tricyclic derivatives result in superior antiviral and pharmacokinetic profiles, J. Med. Chem. 56(2013) 1124-1135. |
| [15] | E. Muraglia, O. Kinzel, C. Gardelli, et al., Design and synthesis of bicyclic pyrimidinones as potent and orally bioavailable HIV-1 integrase inhibitors, J. Med. Chem. 51(2008) 861-874. |
| [16] | X.B. Chen, D.D. Zhu, X.Y. Wang, S.J. Yan, J. Lin, Cascade reaction synthesis of multisubstituted bicyclic pyridone derivatives, Tetrahedron 69(2013) 9224-9236. |
| [17] | M. Tomizawa, N.J. Zhang, K.A. Durkin, M.M. Olmstead, J.E. Casida, The neonicotinoid electronegative pharmacophore plays the crucial role in the high affinity and selectivity for the Drosophila nicotinic receptor:an anomaly for the nicotinoid cation-π interaction model, Biochemistry 42(2003) 7819-7827. |
| [18] | X.S. Shao, W.W. Zhang, Y.Q. Peng, et al., cis-Nitromethylene neonicotinoids as new nicotinic family:synthesis, structural diversity, and insecticidal evaluation of hexahydroimidazo[1,2-α]pyridine, Bioorg. Med. Chem. Lett. 18(2008) 6513-6516. |
| [19] | X.S. Shao, H.Y. Lu, H.B. Bao, et al., The mode of action of a nitroconjugated neonicotinoid and the effects of target site mutation Y151S on its potency, Insect Biochem. Mol. Biol. 41(2011) 440-445. |
| [20] | X.S. Shao, Z. Li, X.H. Qian, X.Y. Xu, Design, synthesis, and insecticidal activities of novel analogues of neonicotinoids:replacement of nitromethylene with nitroconjugated system, J. Agric. Food Chem. 57(2009) 951-957. |
| [21] | X.S. Shao, P.W. Lee, Z.W. Liu, et al., cis-Configuration:a new tactic/rationale for neonicotinoid molecular design, J. Agric. Food Chem. 59(2011) 2943-2949. |
| [22] | X.S. Shao, H. Fu, X.Y. Xu, et al., Divalent and oxabridged neonicotinoids constructed by dialdehydes and nitromethylene analogues of imidacloprid:design, synthesis, crystal structure, and insecticidal activities, J. Agric. Food Chem. 58(2010) 2696-2702. |
| [23] | K. Moriya, K. Shibuya, Y. Hattori, et al., 1-(6-Chloronicotinyl)-2-nitroimino-imidazolidines and related compounds as potential new insecticides, Biosci. Biotechnol. Biochem. 56(1992) 364-365. |
 2016, Vol.27
2016, Vol.27