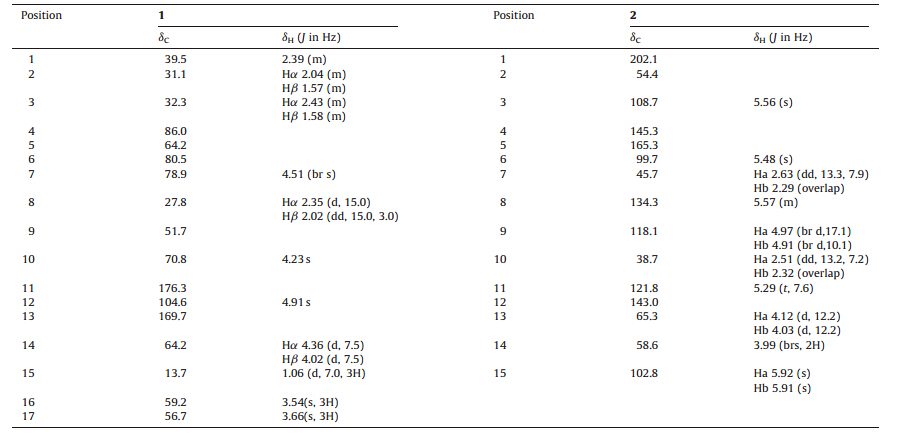
Illicium henryi is a toxic shrub,primarily distRibuted throughout southern China and is used for the tReatment of rheumatism [1]. Several sesquiterpene lactones,lignans and flavonoids from this plant were reported [2, 3, 4, 5, 6, 7, 8]. In our studies,novel prenylated C6- C3 compounds and sesquiterpene lactones with antioxidant and neurotoxicant activities were isolated from the roots of this plant [9, 10, 11]. As a result of our continued searches for the bioactive constituents of this plant,one new sesquiterpene compound, namely,illihenlactone A (1),and one new prenylated C6-C3 compound,illihenryione H (2),along with three known sesquiterpenes (3-5),including pseudoanisatin [12],abscisin [13], phaseic acid [13],(Fig. 1) were isolated from the EtOAc soluble fraction of the EtOH extRact. Herein,we report the isolation and structural elucidation of compounds 1-5.
|
Download:
|
| Fig. 1. Structures of compounds 1–5. | |
Melting points were measured on a XT-5B micromelting point apparatus and were uncorrected. Optical rotations were measured with a Perkin-Elmer 241 automatic digital polarimeter. CD spectRa were measured on a JASCO J-810 spectRopolarimeter with a 0.1 cm cell at room temperature under the following conditions: speed,200 nm/min; time constant,1 s; and bandwidth,2.0 nm. IR spectRa were recorded on a Nicolet 5700 FT-IR spectRometer with a microscope tRansmission method. NMR spectRa were obtained on an INOVA-500 spectRometer. ESIMS were measured on an Agilent 1100 Series LC/MS tRap mass spectRometer. HRESIMS data were recorded using an Agilent 6250 Accurate-Mass Q-TOF LC/MS spectRometer. Preparative HPLC was performed on a Shimadzu LC-6AD instRument with an SPD-10A detector using a YMC-Pack ODS-A column (250 mm × 20 mm,5 mm). Silica gel (160-200,200-300 mesh, Qingdao Marine Chemical Factory,China) and ODS (50 mm, Merck) were used for column chromatography (CC). TLC was performed with precoated Si gel GF254 glass plates. Spots were visualized under UV light or by spraying with 10% H2SO4 in EtOH-H2O (95:5,v/v) followed by heating.
2.2. Plant materialStems of I. henryi were collected in Guangxi Province,China,in August 2009 and identified by Prof. Song-ji Wei from Guangxi University of tRaditional Chinese Medicine. A voucher specimen (ID-21974) was deposited in the Herbarium of the Department of Medicinal Plants,Institute of Materia Medica,Chinese Academy of Medical Sciences.
2.3. ExtRaction and isolationStems of I. henryi (10 kg) were air-dried,ground,and extRacted (2 h each) with EtOH-H2O (3× 30 L,95:5,v/v) by refluxing (90- 95 °C). The combined EtOH extRacts were evaporated to near dryness under vacuum. The resulting residue (400 g) was absorbed on kieselguhr (800 g) and then successively extRacted with petRoleum ether,EtOAc,and MeOH. The EtOAc (58 g) extRact was subjected to a silica gel column (80 cm × 8 cm,200 - 300 mesh) eluted with a CH2Cl2/MeOH (50:1→ 1:1,v/v) gradient system to yield four fractions,A1-A4.
Fraction A2 (8 g) was loaded over an ODS column and eluted with MeOH-H2O (10:90 → 60:40) to afford two fractions,A2B1- A2B2. Fraction A2B1 (500 mg) was purified using a semi-preparative HPLC [MeCN-H2O (40:60)] to yield compounds 1 (50 mg, tR = 35 min) and 5 (10 mg,tR = 32 min). By following the same procedure,compounds 2 (10 mg,tR = 40 min) and 4 (5 mg, tR = 44 min) were obtained from A2B2 (50 mg). Fraction A2B2 (200 mg) was purified by semi-preparative HPLC [MeCN-H2O (35:65)] to yield compounds 3 (30 mg,tR = 36 min).
Illihenlactone A (1): Colorless crystal,mp 138 °C,[α]D20-33.1 (c 0.51 MeOH); IR (cm-1): nmax 3460,3381,2965,2946,2871,1838, 1721,1369,1189,1130,1111,1064,927; For 1H NMR and 13C NMR spectRoscopic data,see Table 1; ESIMS m/z 373 [M+H]+, 395 [M+Na]+,371 [M+H]+; HRESIMS m/z 371.1345 [M-H]- (calcd. for C17H23O9,371.1348).
| Table 1 1H NMR (500 MHz) and 13C NMR (125 MHz) data of compounds 1 in CDOD3 and 2 in acetone-d6. |
Illihenryione H (2): White amorphous powder,[α]D20 + 18.2 (c 0.50 MeOH); CD (MeOH)δε240-0.75,δε302 0.77; UV (MeOH) λmax (log ε): 270 (1.00),220 (0.40); IR (cm-1):νmax 3367,3078,2923, 2852,1678,1621,1603,1415,1391,1229,1203,1134,1015,948, 834; For 1H NMR and 13C NMR spectroscopic data,see Table 1; ESIMS m/z 279 [M+H]+,301 [M+Na]+; HRESIMS m/z 279.1227 [M+H]+ (calcd. for C15H19O5,279.1227).
The 1H NMR and 13C NMR spectra of compound 1 and compound 2 are in the Supporting information.
2.4. Anti-inflammatory activity assayOn the basis of reported procedures [14],the anti-inflammatory activities of compounds 1-5 were assayed by measuring the inhibition of the platelet-activating factor induced release of β-glucuronidase from rat polymorphonuclear leukocytes in vitro.
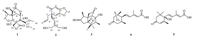
3. Results and discussionCompound 1 was a colorless crystal,and its molecular formula was established as C17H24O9 by HRESIMS (371.1345 [M-H]-) and NMR data (Table 1). Its IR spectrum showed absorptions for hydroxyl (3460 cm-1) and ester carbonyl (1721 cm-1) moieties. The NMR data (Table 1) of 1 were similar to those of veranisatin A, except that a CH2(12)-OCH3(16) group [δH 4.05 (d,1H,J = 11.0,H- 12a),3.05 (d,1H,J = 11.0,H-12b),3.41(s,3H,H-16); δC 73.4 (C-12), 60.5 (C-16)] in veranisatin A was replaced by a hemiacetal moiety [δH 3.66 (s,3H,H-17),3.54 (s,3H,H-16),4.91 (s,1H,H-12); δC 56.7 (C-17),59.2 (C-16),104.6 (C-12)] in 1,and this was further confirmed by the HMBC correlations from H3-16,H3-17 to C-12 and from H-12 to C-5,C-6 and C-7. NOESY spectRum showed crosspeaks between H3-15 and H-10 and between H-12 and H2-14. (Fig. 2) Therefore,the structure of 1 was elucidated as shown in Fig. 1 and named illihenlactone A (1).
|
Download:
|
Fig. 2. The HMBC ( ), H-HCOSY (
), H-HCOSY (  ) and NOESY
) and NOESY  correlations of compound 1.
correlations of compound 1.
|
|
Compound 2 was assigned as one of prenylated C6-C3 derivatives because it possessed characteristic UV and IR spectroscopic data [15]. The molecular formula of 2,C15H18O5,was established by HRESIMS (m/z 279.1227 [M+H]+. calcd. for 279.1227) and NMR data, which indicated 7 degrees of unsaturation. The 1H NMR data of 2 were similar to those of 4-allyl-4-(3-methylbut-2-enyl)-1,2- methylenedioxycyclohexa-2,6-dien-5-one [16],except for the absence of two methyl signals and the presence of two oxygenated methylene resonances. The above data indicated that C-13 and C-14 in 2 were two hydroxymethyl groups instead of the two methyl groups observed in 4-allyl-4-(3-methylbut-2-enyl)-1,2- methylenedioxycyclohexa-2,6-dien-5-one,and this was further determined by the HMBC correlations from H2-13 to C-14,C-12, and C-11 and from H2-14 to C-13,C-12,and C-11. The CD spectrum showed a positive Cotton effect at 302 nm,indicating that the configuration of C-2 was R (Fig. 3) [17],and compound 2 was named illihenryione H (2).

|
Download:
|
| Fig. 3. The CD spectrum of compound 2. | |
The anti-inflammatory effects of 1-5 were determined. Compounds 1-5 were assessed by measuring the inhibitory ratios for β-glucuronidase release induced by platelet-activating factor (PAF) in rat polymorphonuclear leukocytes (PMNS) in vitRo [14]. The inhibitory ratio of 2 was 30.3% at a concentRation of 10 mmol/L. Ginkgolide B,with an inhibitory ratio of 81.1% at 10 mmol/L,was used as a positive contRol (Table 2).
| Table 2 Inhibition effect of compounds 1–5 on PAF-induced release of β-glucuronidase from rat PMNs (in vitro). |
The genus Illicium is a rich source of prenylated C6-C3 compounds and sesquiterpene lactones. Prenylated C6-C3 compounds,also named phytoquinoids,are considered to be characteristic constituents. From a chemical viewpoint,these compounds belong to cyclic prenylated tetrahydrofurano-type,cyclic prenylated tetrahydropyrano-type,cyclic prenylated bridge-type C6- C3 and non-cyclic prenylated C6-C3-type compounds as reported [17]. In this paper,we described the isolation and identification of two new compounds,illihenlactone A (1) and illihenryione H (2) which are classified as secoprezizaane-type and non-cyclic prenylated C6-C3-type compounds,respectively. Biological evaluation indicated that compound 2 showed weak inhibitory activity of b-glucuronidase release from rat PMNs induced by PAF.
AcknowledgmentsThis project was supported by the Natural Science Foundation of China (No. 21132009) and the National Science and Technology Project of China (No. 2012ZX09301002-002). We are grateful to the Department of InstRumental Analysis,Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College for measuring the IR,UV,NMR,MS and CD spectra.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.09.007.
| [1] | J.S. Liu, Q.R. Zhou, The toxic principle of Illicium henryi Diels and structure of 6- deoxypseudoanisatin, Acta Pharm. Sin. 23 (1988) 221-223. |
| [2] | J.F. Liu, Z.Y. Jiang, Q. Zhang, et al., Henrylactones A-E and Anti-HBV constituents from Illicium henryi, Plant. Med. 76 (2010) 152-158. |
| [3] | J.F. Liu, X.M. Zhang, Y. Shi, et al., Chemical constituents from rhizomes of Illicium henryi, Chin. J. Chin. Mater. Med. 35 (2010) 2281-2284. |
| [4] | J.F. Liu, Z.Y. Jiang, C.A. Geng, et al., Two new lignans and Anti-HBV constituents from Illicium henryi, Chem. Biodivers. 8 (2011) 692-698. |
| [5] | T.F. Song, W.D. Zhang, X.H. Xia, et al., Two new acorane sesquiterpenes from Illicium henryi, Arch. Pharm. Res. 32 (2009) 1233-1236. |
| [6] | W.J. Xiang, L. Ma, L.H. Hu, Neolignans and flavonoids from the root bark of Illicium henryi, Fitoterapia 81 (2010) 1228-1231. |
| [7] | D.L. Xie, S. Wang, Z.W. Cheng, Y.S. Song, D.Y. Kong, Analysis of flavonoids in the root-cortex of Henry anisetree (Illicium henryi), Zhongcaoyao 21 (1990) 447-449. |
| [8] | Y.D. Chen, A. Gao, J. Gong, et al., An overview of pharmaceutical research on Illicium henryi Diels, J. Anhui Agric. Sci. 39 (2011) 8376-8377. |
| [9] | P.Y. Zhuang, G.J. Zhang, X.J. Wang, et al., Prenylated C6-C3 compounds from the roots of Illicium henryi, Phytochemistry 86 (2013) 176-183. |
| [10] | P.Y. Zhuang, S.G. Ma, G.J. Zhang, et al., New prenylated C6-C3 compounds from the roots of Illicium henryi, Phytochem. Lett. 6 (2013) 444-448. |
| [11] | P.Y. Zhuang, G.J. Zhang, X.J. Wang, et al., Novel sesquiterpenoid glycosides and sesquiterpenes from the roots of Illicium henryi, Plant. Med. 79 (2013) 1453-1460. |
| [12] | I. Kouno, N. Baba, M. Hashimoto, et al., A new sesquiterpene lactone and its glucoside from the pericarps of Illicium majus, Chem. Pharm. Bull. 37 (1989) 2427-2430. |
| [13] | L.I. Zaharia, Y.Z. Gai, K.M. Nelson, S.J. Ambrose, S.R. Abrams, Oxidation of 8'- hydroxy abscisic acid in Black Mexican Sweet maize cell suspension cultures, Phytochemistry 65 (2004) 3199-3209. |
| [14] | W.Z. Tang, S.G. Ma, S.S. Yu, et al., Rearranged prenylated C6-C3 compounds and a highly oxygenated seco-prezizaane-type sesquiterpene from the stem bark of Illicium oligandrum, J. Nat. Prod. 72 (2009) 1017-1021. |
| [15] | K. Yakushijin, T. Tohshima, R. Suzuki, et al., Studies on the constituents of the plants of Illicium species. Ⅱ. Structures of phenolic components, Chem. Pharm. Bull. 31 (1983) 2879-2883. |
| [16] | J. Kang, R.Y. Chen, D.Q. Yu, Five new diels-alder type adducts from the stem and root bark of Morus mongolica, Plant. Med. 72 (2006) 52-59. |
| [17] | S.G. Ma, W.Z. Tang, Y.X. Liu, et al., Prenylated C6-C3 compounds with molecular diversity from the roots of Illicium oligandrum, Phytochemistry 72 (2011) 115-125. |
 2015, Vol.26
2015, Vol.26