In recent years,considerable attention has been focused on energetic materials based on metastable intermolecular composites (MICs) [1]. In particular,much effort has been devoted to the nano-MICs owing to their negligible gas generation,high heat production [2],high energy density,and high burning rate [3, 4]. Among the nano-MICs,Al/Fe2O3 composite is an important nanoenergetic material that has attracted special attention since it is widely used as an additive to propellants and high explosives, free standing heat sources,airbag ignition materials,hardware destruction devices,and welding torches [5]. The Al/Fe2O3 system contains a metal fuel aluminum (Al) that is traditionally used as the primary fuel because of its high density,efficient catalytic performance [6] and low cost,and one metal oxide (Fe2O3) that led to enhanced reactivity [7, 8, 9, 10]. The composites with nanostructures also allow for better reactivity control by varying parameters such as morphology and particle size [11].
The Al/Fe2O3 composite can be synthesized using a number of methods,including sol-gel [12, 13, 14],self-assembly [15, 16, 17] and mechanical processing (ultrasonic dispersion method [18],powder mixing). Electrospinning is a rather simple and highly versatile technique to generate multifunctional ultrathin fibers from various polymers,polymer blends,and polymer nanoparticles. Most recently,it has been employed to create nitrocellulose/Al [19] and nitrocellulose/Al/CuO [3] energetic fibers as a potential propellant.
In this paper,we present the fabrication of Al/Fe2O3 composites with different morphologies based on a surfactant (polyvinyl pyridine,PVP) in a mixture of N,N-dimethylformamide (DMF) and 2-propanol with the electrospinning method. The Al/Fe2O3 composites were characterized by a combination of experimental techniques including X-ray diffraction (XRD),energy dispersive spectrometer (EDS),scanning electron microscopy (SEM),and transmission electron microscopy (TEM). We hope this work can form a foundation to use a rather simple technique for the preparation of morphology-controlled MICs,propellants,explosives,and pyrotechnics in the future.
2. ExperimentalThe electrospinning apparatus used in this work consisted of a single stainless steel capillary tube maintained at a potential of 15 kV with respect to a ground plate positioned about 15 cm from the capillary tip. The electrospun composites were prepared as follows: First,2.0 g of Fe(NO3)3.9H2O (Tongguang Chemical Reagent Co.,Ltd.) and 2.0 g of PVP (Mn = 1,300,000,Aldrich Chemical Company) were dissolved. Then,0.4 g of Al (80-200 nm, Jiaozuo nanomaterial Company) was dispersed into the solution by magnetic stirring in 4 h. The comparison was prepared from a solution consisting of 2.0 g of PVP dissolved in a mixed solvent of DMF and 2-propanol (v/v = 10.6 mL/8.4 mL). And then 0.8 g of Fe2O3 (30-50 nm,Aldrich Chemical Company) and 0.4 g of Al were dispersed into the solution by magnetic stirring in 4 h. The electrospun composite nanofibers were calcinated at 500 °C for 3 h in N2 at a rate of 5 °C/min,and finally Al/Fe2O3 composites were obtained [20].
XRD spectra were obtained by a PANalytical X’Pert PROMPD powder diffraction apparatus. EDS and SEM were taken using a S4800 Field-Emission Scanning Electron Microscope Hitachi. TEM was recorded on a JEM 1200EX H-700 Microscope. The sample was dispersed into ethanol by the ultrasonic method (5 mg/mL). A drop of the sample dispersion was dropped on to a copper grid (200 mesh) with carbon film. Then the copper grid coated with the sample was allowed to dry at room temperature.
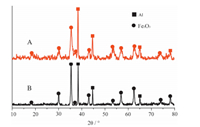
3. Results and discussionThe crystal structure and phase composition of the electrospun Al/Fe2O3 composites after calcinated at 500 °C were confirmed by the XRD analysis (Fig. 1). The apparent and sharp reflection peaks correspond to those given in the Joint Committee on Powder Diffraction Standards (JCPDS) card 39-1346 of Fe2O3 and JCPDS card 65-2869 of Al. The XRD patterns reveal no unknown crystalline phases and impurities in the Al/Fe2O3 composites. The XRD results of Al/Fe2O3 composites obtained from Fe(NO3)3.9H2O/PVP/Al are coincided with those from Fe2O3/PVP/ Al. It indicates that the Al/Fe2O3 composites could be obtained from Fe(NO3)3.9H2O/PVP/Al successfully.
|
Download:
|
| Fig. 1. XRD spectra of the electrospun Al/Fe2O3 composites after calcinated at 500 °C. (A) Obtained from Fe(NO3)3.9H2O/PVP/Al; (B) obtained from Fe2O3/PVP/Al. | |
The SEM images of electrospun composites are shown in Fig. 2. Fig. 2A shows that all of the electrospun composites are fibers in morphology with nanoscale and the surface is smooth. After calcination at 500 °C,the surface of fibers becomes rough,but the morphology is unchanged (Fig. 2B). In comparison,the composites from Fe2O3/PVP/Al also are fibers in morphology with nanoscale before calcinated (Fig. 2C). However after calcination at 500 °C,the fiber-like composites become spherical particles in nanosize and the spherical particles seem somewhat aggregated (Fig. 2D). The morphology of electrospun composites is changed. It is attributed to the disordered spherical Al and Fe2O3 aggregates when the PVP skeleton is removed by calcination.

|
Download:
|
| Fig. 2. SEM images of the electrospun composites. (A) Fe(NO3)3.9H2O/PVP/Al; (B) Fe(NO3)3.9H2O/PVP/Al after calcinated at 500 °C; (C) Fe2O3/PVP/Al; (D) Fe2O3/PVP/Al after calcinated at 500 °C. | |
In order to obtain the distribution of Al and Fe2O3,the EDS of the electrospun Al/Fe2O3 composites after calcination at 500 °C is investigated. The peaks at about 2.2 keV,which are signals of gold in all the samples,are treated by gold sputtering to increase their conductivity before the test of SEM-EDS. The element C contributes to the residual carbon of calcinated PVP. The O,Fe and Al peaks are in accordance with the XRD pattern. The elements O,Fe and Al are distributed evenly in the pictures,which indicated that the Al is in touch with Fe2O3 completely (Fig. 3).

|
Download:
|
| Fig. 3. EDS of the electrospun Al/Fe2O3 composites after calcinated at 500 °C. (A) Obtained from Fe(NO3)3.9H2O/PVP/Al; (B) obtained from Fe2O3/PVP/Al. | |
The morphology and microstructure of the as-synthesized samples are also characterized by TEM. Images of the electrospun Al/Fe2O3 system in Fig. 4 clearly show that the morphologies of electrospun Al/Fe2O3 system obtained from different materials are distinct. It shows that fibers and some spherical particles aggregate in line (Fig. 4A). It is attributed to the size of the Al particles. When the diameter of Al is bigger than the fibers,the surface of fiber presents protuberance,which resembles a line consisted of spherical particles. The Al and Fe2O3 aggregate is disordered as shown in Fig. 4B. The TEM result is in accordance with the SEM pattern of Al/Fe2O3 composites after calcination at 500 °C.

|
Download:
|
| Fig. 4. TEM of the electrospun Al/Fe2O3 composites after calcinated at 500 °C. (A) Obtained from Fe(NO3)3.9H2O/PVP/Al; (B) obtained from Fe2O3/PVP/Al. | |
Scheme 1 shows a schematic presentation of the preparation process of the Al/Fe2O3 composites. The fiber-like Al/PVP/ Fe(NO3)3.9H2 composites are obtained by electrospinning from the electrospinning dispersion of which the Al is dispersed in a PVP/Fe(NO3)3.9H2 solution before calcination. After calcination at 500 °C,the Al/PVP/Fe(NO3)3.9H2 composites transform to Al/ Fe2O3 composites. Although the chemical composition has changed,the morphologies of the two kinds of composites are still fibrous due to the fact that both contain a skeleton. The former has a PVP/Fe(NO3)3.9H2 skeleton and the latter has a Fe2O3 skeleton. During the process of calcination,the morphology of the formed Fe2O3 skeleton is controlled by PVP,which is attributed to the complete contact between Fe(NO3)3.9H2 and PVP [20]. For comparison,fiber-like Al/PVP/Fe2O3 composites are acquired by electrospinning from the electrospinning dispersion of which the spherical Al and Fe2O3 particles are dispersed in a PVP solution. After calcination at 500 °C,the fiber-like morphology is not observed and the spherical Fe2O3 and Al aggregate. The removal of the existing PVP skeleton or the formation of a new skeleton is not observed at high temperatures,which are responsible for these changes. These are in accordance with the results of SEM and TEM.

|
Download:
|
| Scheme. 1. Schematic presentation of the preparation process of Al/Fe2O3 composites. | |
In summary,we have reported a novel way to prepare Al/Fe2O3 composites with different morphologies via an electrospinning approach. When the conditions of the materials changed,Al/Fe2O3 composites with different morphologies were obtained with the same electrospinning and calcination methods. The morphologycontrolled Al/Fe2O3 composites obtained by the simple electrospinning approach may have the potential for the preparation of propellants,explosives,and pyrotechnics in the future.
| [1] | K.S. Martirosyana, Nanoenergetic gas-generators: principles and applications, J. Mater. Chem. 21 (2011) 9400-9405. |
| [2] | L.L. Wang, Z.A. Munir, Y.M. Maximov, Thermite reactions: their utilization in the synthesis and processing of materials, J. Mater. Sci. 28 (1993) 3693-3708. |
| [3] | S. Yan, G.Q. Jian, M.R. Zachariah, Electrospun nanofiber-based thermite textiles and their reactive properties, ACS Appl. Mater. Interfaces 4 (2012) 6432-6435. |
| [4] | J.J. Granier, K.B. Plantier, M.L. Pantoya, The role of the Al2O3 passivation shell surrounding nano-Al particles in the combustion synthesis of NiAl, J. Mater. Sci. 39 (2004) 6421-6431. |
| [5] | U. Teipel, Energetic Materials: Particle Processing and Characterization, Viley- VCH Verlag, Weinheim, 2005. |
| [6] | L.P. Zhou, J. Xu, X.Q. Li, F. Wang, Metal oxide nanoparticles from inorganic sources via a simple and general method, Mater. Chem. Phys. 97 (2006) 137-142. |
| [7] | M.L. Pantoya, J.J. Granier, Combustion behavior of highly energetic thermites: nano versus micron composites, Propellants Explos. Pyrotech. 30 (2005) 53-62. |
| [8] | K. Sullivan, G. Young, M.R. Zachariah, Enhanced reactivity of nano-B/Al/CuO MIC's, Combust. Flame 156 (2009) 302-309. |
| [9] | R.W. Armstrong, B. Baschung, D.W. Booth, M. Samirant, Enhanced propellant combustion with nanoparticles, Nano Lett. 3 (2003) 253-255. |
| [10] | B.S. Bockmon, M.L. Pantoya, S.F. Son, B.W. Asay, J.T. Mang, Combustion velocities and propagation mechanisms of metastable interstitial composites, J. Appl. Phys. 98 (2005) 064903. |
| [11] | L. Zhou, N. Piekiel, S. Chowdhury, M.R. Zachariah, Time-resolved mass spectrometry of the exothermic reaction between nanoaluminum and metal oxides: the role of oxygen release, J. Phys. Chem. C 114 (2010) 14269-14275. |
| [12] | T.M. Tillotson, A.E. Gash, R.L. Simpson, et al., Nanostructured energetic materials using sol-gel methodologies, J. Non-Cryst. Solids 285 (2001) 338-345. |
| [13] | M.A. Aegerter, N. Leventis, M.M. Koebel, Aerogels Handbook, Springer-Verlag, New York, 2011. |
| [14] | K.B. Plantier, M.L. Pantoya, A.E. Gash, Combustion wave speeds of nanocomposite Al/Fe2O3: the effects of Fe2O3 particle synthesis technique, Combust. Flame 140 (2005) 299-309. |
| [15] | J.M. Slocik, C.A. Crouse, J.E. Spowart, R.R. Naik, Biologically tunable reactivity of energetic nanomaterials using protein cages, Nano Lett. 13 (2013) 2535-2540. |
| [16] | J.L. Cheng, H.H. Hng, H.Y. Ng, P.C. Soon, Y.W. Lee, Synthesis and characterization of self-assembled nanoenergetic Al-Fe2O3 thermite system, J. Phys. Chem. Solids 71 (2010) 90-94. |
| [17] | J.L. Cheng, H.H. Hng, Y.W. Lee, S.W. Du, N.N. Thadhani, Kinetic study of thermaland impact-initiated reactions in Al-Fe2O3 nanothermite, Combust. Flame 157 (2010) 2241-2249. |
| [18] | N.N. Zhao, C.C. He, J.B. Liu, et al., Dependence of catalytic properties of Al/Fe2O3 thermites on morphology of Fe2O3 particles in combustion reactions, J. Solid State Chem. 219 (2014) 67-73. |
| [19] | L. Xie, Z.Q. Shao, W.J. Wang, F.J. Wang, Preparation of AlNPs/NC composite nanofibers by electrospinning, Integr. Ferroelectr. 127 (2011) 184-192. |
| [20] | X. Chen, K.M. Unruh, C.Y. Ni, et al., Fabrication, formation mechanism, and magnetic properties of metal oxide nanotubes via electrospinning and thermal treatment, J. Phys. Chem. C 115 (2011) 373-378. |
 2015, Vol.26
2015, Vol.26 


