b Department of Chemistry, Georgia State University, Atlanta, GA 30303, USA;
c Department of Microbiology, Faculty of Pharmacy, Mansoura University, Mansoura 35516, Egypt;
d Department of Microbiology, College of Pharmacy, Taibah University, Almadinah Almunawwarah 344, Saudi Arabia
The traditional antibacterial agents either kill bacteria (bactericidal) or inhibit their growth (bacteriostatic). Typically,the targets for the conventional antibiotics are the essential cellular processes such as bacterial cell wall biosynthesis,bacterial protein synthesis, and bacterial DNA replication and repair [1]. The eventual growth arrest and cell death can be followed by rapid expansion of resistant subpopulations,making subsequent treatment difficult or impossible [2]. Therefore,new antibacterial strategies are required. An alternative to killing or inhibiting growth of pathogenic bacteria is the specific attenuation of bacterial virulence,which could be attained by targeting key regulatory systems that mediate the expression of virulence factors. One of the target regulatory systems is quorum sensing (QS) [1]. QS is a phenomenon used by bacteria for coordination of population-wide phenotypes,such as expression of virulence genes,antibiotic resistance and biofilm formation. QS disruption is one of the emerging anti-virulence strategies that promises a lower risk of resistance development [3]. Many quorum quenching methods have been developed against various clinically significant bacterial pathogens [4].
The benzothiazole nucleus is a unique scaffold for further molecular exploration to synthesize novel compounds. Literature survey revealed that benzothiazole analogs are associated with diverse pharmacological effects,including antimicrobial activity [5, 6, 7, 8, 9]. In addition,benzothiazoles incorporating pyrazole moiety demonstrated remarkable antimicrobial activity [10, 11]. On the same line,benzothiazoles incorporating isatin moiety have received considerable attention owing to their diverse chemotherapeutic potentials,including antimicrobial activity [12, 13]. In addition,various Schiff bases of 2-hydrazinobenzothiazole derivatives (Fig.1) were previously synthesized and screened for their antimicrobial activity [14, 15, 16]. Some of these derivatives displayed promising activity.

|
Download:
|
| Fig. 1. Schiff bases of 2-hydrazinobenzothiazole derivatives with reported antimicrobial activity. | |
Therefore,we found it interesting to design new compounds within the scope of synthetic procedures using the benzothiazole scaffold followed by suitable modifications to generate new series of compounds with expected antimicrobial activity. The manipulation strategy embraces the incorporation of pyrazole,isatin and arylidene moieties into the benzothiazole ring in order to verify the importance of these moieties for the antimicrobial activity (Fig.2).

|
Download:
|
| Fig. 2. Designed strategy of the titled compounds. | |
A general approach for the synthesis of the designed compounds is outlined in Scheme 1. The starting compound,2- amino-6-fluorobenzothiazole (1) was reacted with hydrazine hydrate in refluxing ethylene glycol in the presence of hydrochloric acid to produce the hydrazine derivative 2 [17]. Refluxing compound 2 with ethyl 3-oxo-2-((2-substituted phenyl)hydrazono)butanoates 3a-e [18] in glacial acetic acid yielded the corresponding pyrazole analogs 4a-e. In addition,the reaction of the key intermediate 2 with the appropriate isatin in ethanol in the presence of glacial acetic acid gave compounds 5a-c. Reaction of 2 with the appropriate aromatic aldehyde in ethanol under microwave irradiation gave the corresponding Schiff bases 6a-r in 64%-82% yields. Moreover,refluxing the hydrazine analog 2 with the appropriate acetophenone in ethanol in the presence of glacial acetic acid furnished compounds 7a-d in 61%-73% yields. The newly synthesized compounds,4a-e,5a-c,6a-r and 7a-d were screened for their in vitro antibacterial activity against two species of Gram-positive bacteria (Staphylococcus aureus and Bacillus cereus) and one Gram-negative bacterium (Escherichia coli) [19, 20]. Antifungal screening against Candida albicans and Aspergillus fumigatus 293 was also performed [20, 21]. The same compounds were examined for their antiquorum-sensing activity against Chromobacterium violaceum ATCC 12472 [22]. Additionally, the in vitro cytotoxicity testing of compounds 4a-e,5a-c,6a-r and 7a-d was performed against cervical cancer (Hela) and kidney fibroblast cancer (COS-7) cell lines adopting MTT assay [23, 24, 25].

|
Download:
|
| Scheme. 1. Synthesis of compounds 4a–e, 5a–c, 6a–r and 7a–d. | |
The synthetic details and related spectra of the compounds as well as their biological testing are deposited in Supporting information.
3. Results and discussion 3.1. ChemistryThe structures of all the synthesized compounds were confirmed by 1H NMR,13C NMR and HRMS. 1H NMR spectra of compounds 4a-e showed a characteristic singlet at δ 2.05- 2.50 ppm for the methyl protons at the 3-position of the pyrazole ring. In the 1H NMR spectra of compounds 6a-r,a singlet at δ 7.95- 8.83 ppm was due to CH5 5N proton. Regarding 1H NMR spectra of compounds 7a-d,methyl protons were observed as a singlet at δ 1.90-2.35 ppm.
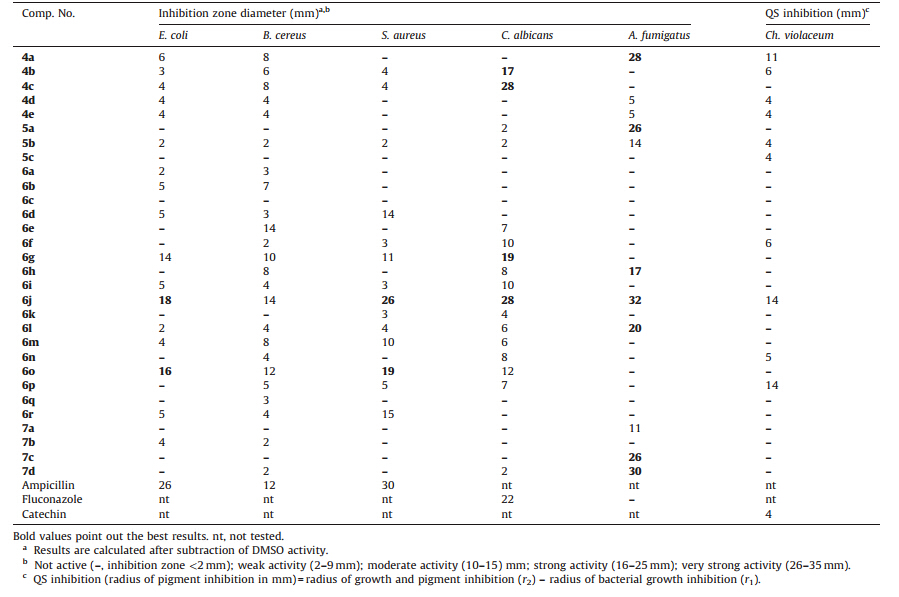
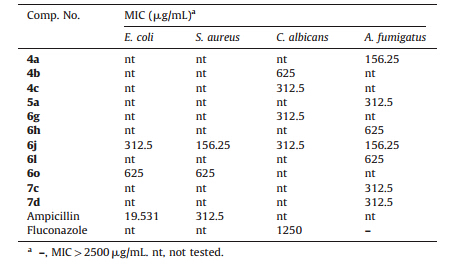
3.2. Biological screeningThe antimicrobial screening results (Table1) were determined by measuring the average diameter of the inhibition zones, expressed in millimeters (mm) [19, 21]. The minimum inhibitory concentration (MIC,mg/mL) of the most active compounds against E. coli,S. aureus,C. albicans and A. fumigatus 293 was carried out by broth microdilution method using 96-multiwell microtiter plates [20]. As shown in the results (Table2),compound 6j showed the highest activity against E. coli with MIC value of 312 mg/mL. Furthermore,compound 6j exhibited good antibacterial activity against S. aureus with MIC value of 156.25 mg/mL. The results are compared to ampicillin as a reference antibacterial agent. Regarding the antifungal activity,compounds 4c,6g and 6j displayed the highest activity against C. albicans with MIC value of 312.5 mg/mL. In addition,compounds 4a and 6j demonstrated strong antifungal activity against A. fumigatus 293 with MIC value of 156.25 mg/mL (Table2). The results are compared to fluconazole as a reference antifungal agent. A. fumigatus 293 was resistant to fluconazole [26]. These observations may promote further development of benzothiazole derivatives and may lead to compounds with potent antibacterial and antifungal activities.
| Table 1 Antimicrobial and antiquorum-sensing activities of compounds 4a–e, 5a–c, 6a–r and 7a–d. |
| Table 2 MIC values of the most active compounds. |
While antibiotics kill or slow down the growth of bacteria, quorum sensing inhibitors (QSIs) or quorum quenchers (QQs) attenuate bacterial virulence and appear to be a promising strategy to control bacterial resistance to antibiotics [27]. Thus,the same compounds were examined for their antiquorum-sensing activity against Ch. violaceum ATCC 12472 [22]. The QS system of Ch. violaceum was used for this assay. QS in this wild type strain of bacteria produces violacein (a purple pigment) in response to autoinducer molecules known as acyl HSLs [28, 29]. Thus,drugs that inhibit acyl HSL-mediated QS activity in Ch. violaceum would prevent the production of this purple pigment. Screening results for their ability to inhibit QS regulated violacein production against Ch. violaceum (based on measuring the radius of pigment inhibition in mm) are presented in Table1 and revealed that compounds 4a, 6j and 6p have moderate antiquorum-sensing activity. The rest of the tested compounds were found to be less active or completely inactive. The results are compared to catechin as a positive control. The results of in vitro cytotoxicity testing against cervical cancer (Hela) and kidney fibroblast cancer (COS-7) cell lines indicated that all tested compounds have IC50 values >50 mmol/L against both cell lines.
3.3. Structure-activity relationship (SAR) studiesCompounds 4a-e: Removal of the 2-chloro substituent from the phenyl ring of compound 4a increased the activity against C. albicans but abolished the activity against A. fumigatus 293 (compound 4b). Moreover,replacement of the 4-nitro substituent in the same compound with 4-chloro substituent resulted in excellent activity against C. albicans (compound 4c) but abolished activity against A. fumigatus 293. Compounds bearing 2,4-disubstituted phenyl and 4- substituted phenyl moieties exhibited stronger activity against C. albicans compared to compounds bearing 2,5-disubstituted phenyl moiety (compounds 4b,c vs. 4d,e).
Compounds 5a-c: Replacement of 2-oxoindolin-3-ylidene moiety (compound 5a) with 5-bromo-2-oxoindolin-3-ylidene (compound 5b) resulted in decreased antifungal activity against A. fumigatus 293,while its replacement with 5-methyl-2-oxoindolin-3-ylidene abolished the antifungal activity against both of the tested fungi (compound 5c).
Compounds 6a-r: Compounds 6a,b bearing (furan-2-yl)methylene moiety were inactive as antifungal agents and revealed weak activity against E. coli and B. cereus. Replacement of (furan-2- yl)methylene moiety with (1H-pyrrol-2-yl)methylene moiety completely abolished the antibacterial activity against E. coli and B. cereus (compound 6c vs. 6a,b). The presence of 2- nitrobenzylidene moiety resulted in acceptable antibacterial activity against the three tested microorganisms as well as antifungal activity against C. albicans (compound 6g),while its replacement with 2-bromobenzylidene or 2-cyanobenzylidene Ch. violaceum (based on measuring the radius of pigment inhibition resulted in decreased antibacterial and antifungal activities (compounds 6i and 6k). Furthermore,the presence of 3-hydroxybenzylidene moiety revealed strong antifungal activity against A. fumigatus 293 (compound 6h). Incorporation of 3,4-dihydroxybenzylidene moiety into the benzothiazole nucleus resulted in promising activity against E. coli,S. aureus,C. albicans and A. fumigatus 293 as well as moderate antiquorum-sensing activity (compound 6j). Replacement of 4-N,N-dimethylaminobenzylidene moiety with 4-N,N-diethylamino-2-hydroxybenzylidene increased the antibacterial activity against the three tested microorganisms but abolished the antifungal activity against A. fumigatus 293 (compound 6m vs. 6l). Replacement of (pyridin- 2-yl)methylene moiety with (3,5-dichloropyridin-4-yl)methylene increased the antibacterial activity against the three tested microorganisms as well as antifungal activity against C. albicans (compound 6o vs. 6n). Incorporation of (isoquinolin-5-yl)methylene moiety into the benzothiazole nucleus improved the antiquorum-sensing activity (compound 6p),while incorporation of (pyren-2-yl)methylene moiety enhanced the antibacterial activity against S. aureus (compound 6r).
Compounds 7a-d: The presence of 2-hydroxy-5-methylphenyl and 4-iodophenyl moieties resulted in promising anifungal activity against A. fumigatus 293 (compounds 7c,d),while incorporation of 5-bromo-2-hydroxyphenyl moiety into the benzothiazole nucleus resulted in moderate antifungal activity against the same microorganism (compound 7a). On the other hand,incorporation of 3-hydroxy-2-methoxyphenyl moiety abolished the antifungal activity against the same microrganism (compound 7b).
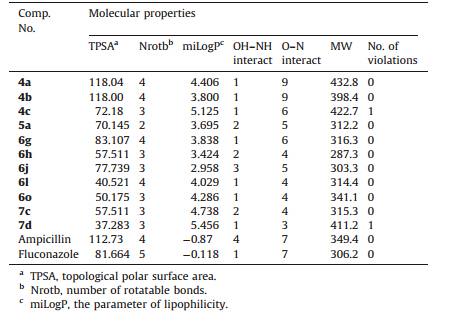
3.4. Molecular properties and drug-likenessA molecular property is a complex balance of various structural features which determine whether a particular molecule is similar to the known drugs. It generally means ‘‘molecules which contain functional groups and/or have physical properties consistent with most of the known drugs’’. Hydrophobicity,molecular size, flexibility and presence of various pharmacophoric features are the main physical properties that influence the behavior of molecules in a living organism. Computational chemists have a wide array of tools and approaches available for the assessment of molecular diversity. Diversity analysis has been shown to be an important ingredient in designing drugs. So,computational sensitivity and structural analyses have been used to study the drug-likeness of the candidate drug. As good bioavailability can be achieved with an appropriate balance between solubility and partitioning properties. Thus,in order to achieve good oral drugs, compounds 4a-c,5a,6g,h,6j,6l,6o and 7c,d which exhibited the highest antibacterial and/or antifungal activity,were analyzed for the prediction of solubility and Lipinski’s rule of five [30] as well as other properties (Tables 3 and 4) for filtering compounds for subsequent synthesis and antimicrobial screening.
| Table 3 Topological polar surface area, number of rotatable bonds and calculated Lipinski’s rule of five for compounds 4a–c, 5a, 6g,h, 6j, 6l, 6o and 7c,d. |
| Table 4 Toxicity risks, drug-likeness and drug score of compounds 4a–c, 5a, 6g,h, 6j, 6l, 6o and 7c,d. |
As a part of our study; the compliance of compounds to the Lipinski’s rule of five was evaluated [30],this simple rule is based on the observation that most biologically active drugs have molecular weight of 500 or less,logP values not higher than 5, hydrogen bond donor sites not higher than 5 and hydrogen bond acceptor sites not higher than 10. In addition,topological polar surface area (TPSA) and number of rotatable bonds have been linked to drug bioavailability [31]. Molecular properties (TPSA, nrotb,miLogP,OH-NH interaction,O-N interaction,molecular weight and number of violations from Lipinski’s rule) of the newly synthesized compounds were calculated using molinspiration software and compared to the values of the standard drugs, ampicillin and fluconazole (Table3).
Topological polar surface area (TPSA) and number of rotatable bonds are two important properties for the prediction of oral bioavailability of drug molecules [32, 33, 34, 35]. TPSA is calculated based on the methodology published by Ertl et al. [35] as the surface areas that are occupied by oxygen and nitrogen atoms and by hydrogen atoms attached to them. Thus,it is closely related to the hydrogen bonding potential of a compound [32, 33, 34, 35]. TPSA has been shown to be a very good descriptor characterizing drug absorption,including intestinal absorption,bioavailability and blood-brain barrier penetration. Molecules with TPSA values around 140 Å 2 or more are expected to exhibit poor intestinal absorption [31]. Results shown in Table3 indicated that all of the analyzed compounds have TPSA values < 140Å 2; thus,they are expected to have good intestinal absorption. Molecules with more than 10 rotatable bonds may have problems with bioavailability [31]. All the tested compounds have 2 to 4 rotatable bonds and they might not have problems with bioavailability (Table3). MiLogP is calculated adopting the methodology developed by Molinspiration as a sum of fragment-based contributions and correction factors (http:// www.molinspiration.com). It has been shown that for the compound to have a reasonable probability of being well absorbed, miLogP value must be in the range of -0.4 to +5 [31]. On this basis, all compounds under investigation (except 4c and 7d) were found to have miLogP values under the acceptable criteria and they are expected to have reasonable oral absorption (Table3). It is worth mentioning that all of the analyzed compounds have one or zero violation of Lipinski’s rule; therefore,they are expected not to have problems with bioavailability (Table3). Molecules violating more than one may have problems with bioavailability [36].
3.4.2. Osiris calculationsToxicity risks (mutagenicity,tumorogenicity,irritancy and reproductive effects) and physicochemical properties (druglikeness and drug score) of compounds 4a-c,5a,6g,h,6j,6l,6o and 7c,d were calculated by the methodology developed by Osiris [32]. The toxicity risk predictor locates fragments within a molecule which indicate a potential toxicity risk. Toxicity risk alerts indicate that the drawn structure may be harmful concerning the risk category specified. From the data presented in Table4,it is obvious that the analyzed compounds are supposed to be non-mutagenic (except 4a and 6g),non-tumorigenic (except 4a,6g and 6l),non-irritating,and with no reproductive effects (except 4a-c).
Drug-likeness is defined as a complex balance of various molecular properties and structural features which indicates whether a particular molecule is similar to the known drugs or not [37]. Osiris program was used for calculating the fragmentbased drug-likeness of compounds 4a-c,5a,6g,h,6j,6l,6o and 7c,d,a positive value indicates that the designed molecule contains fragments which are frequently present in commercial drugs. Results shown in Table4 indicated that compounds 4c,5a and 6j have higher drug-likeness values than the standard drug, fluconazole. The drug score combines drug-likeness,miLogP, solubility,molecular weight and toxicity risks in one handy value that may be used to judge the compound’s overall potential to qualify for a drug [32]. A value of 0.5 or more makes the compound a promising lead for future development of safe and efficient drugs. The overall drug score values for compounds 4a-c,5a,6g,h,6j,6l, 6o and 7c,d were calculated and compared to that of the standard drugs,ampicllin and fluconazole. Compounds 5a,6h and 6j possess good drug score values (Table4).
4. ConclusionIn a summary,compounds 6j and 6o are good antibacterial agents. Compounds 4c and 6j showed the highest antifungal activity against C. albicans. In addition,compounds 4a and 6j displayed the best antifungal activity against A. fumigatus 293. Furthermore,compounds 4a,6j and 6p showed moderate antiquorum-sensing activity. These active compounds were proved to be good scaffolds for the development of new potent antibacterial and antifungal agents.
Conflict of interestThe authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
AcknowledgmentsThe authors thank Professor Binghe Wang at Georgia State University,USA,for providing all required facilities and carrying out the spectral analyses as well as the cytotoxicity testing.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.09.004.
| [1] | T. Defoirdt, N. Boon, P. Bossier, Can bacteria evolve resistance to quorum sensing disruption?, PLoS Pathog. 6 (7) (2010) e1000989, http://dx.doi.org/10.1371/journal. ppat.1000989. |
| [2] | G. Werner, B. Strommenger, W. Witte, Acquired vancomycin resistance in clinically relevant pathogens, Future Microbiol. 3 (2008) 547-562. |
| [3] | L. Cegelski, G.R. Marshall, G.R. Eldridge, S.J. Hultgren, The biology and future prospects of antivirulence therapies, Nat. Rev. Microbiol. 6 (2008) 17-27. |
| [4] | S.B. Tay, W.S. Yew, Development of quorum-based anti-virulence therapeutics targeting Gram-negative bacterial pathogens, Int. J. Mol. Sci. 14 (2013) 16570- 16599. |
| [5] | S. Agarwal, D.K. Agarwal, N. Gautam, K. Agarwal, D.C. Gautam, Synthesis and in vitro antimicrobial evaluation of benzothiazole incorporated thiazolidin-4-ones derivatives, J. Korean Chem. Soc. 58 (2014) 33-38. |
| [6] | M.K. Singha, R. Tilak, G. Nath, S.K. Awasthi, A. Agarwal, Design, synthesis and antimicrobial activity of novel benzothiazole analogs, Eur. J. Med. Chem. 63 (2013) 635-644. |
| [7] | S. Yilmaz, I. Yalcin, F. Kaynak-Onurdag, et al., Synthesis and in vitro antimicrobial activity of novel 2-(4-(substituted-carboxamido)benzyl/phenyl)benzothiazoles, Croat. Chem. Acta 86 (2013) 223-231. |
| [8] | P. Patel, J. Pillai, N. Darji, P. Patel, B. Patel, Design, synthesis and characterization of novel molecules comprising benzothiazole and sulphonamide linked to substituted aryl group via azo link as potent antimicrobial agents, Int. J. Drug Res. Technol. 2 (2012) 289-296. |
| [9] | K.A. Goud, J.N. Narendra, L.V.G. Nargund, et al., Synthesis of novel benzothiazoles for anti-bacterial activity, Der Pharm. Chem. 4 (2012) 1408-1414. |
| [10] | E.S. Darwish, A.M. Abdel Fattah, F.A. Attaby, O.N. Al-Shayea, Synthesis and antimicrobial evaluation of some novel thiazole, pyridone, pyrazole, chromene, hydrazone derivatives bearing a biologically active sulfonamide moiety, Int. J. Mol. Sci. 15 (2014) 1237-1254. |
| [11] | O.M.O. Habib, H.M. Hassan, E.B. Moawad, A. El-Mekabaty, Reactivity of oxazolone derivative towards nitrogen and carbon nucleophilic reagents: applications to the synthesis of new heterocycles, Int. J. Modern Org. Chem. 2 (2013) 11-25. |
| [12] | K.O. Badahdah, H.M. Abdel Hamid, S.A. Noureddin, Functionalized 2-hydrazinobenzothiazole with isatin and some carbohydrates under conventional and ultrasound methods and their biological activities, J. Heterocyclic Chem. 52 (2015) 67-74. |
| [13] | A. Aboelmagd, I.A.I. Ali, E.M.S. Salem, M. Abdel-Razik, Synthesis and antifungal activity of some 2-benzothiazolylthioacetyl amino acid and peptide derivatives, ARKIVOC ix (2011) 337-353. |
| [14] | G. Alang, R. Kaur, G. Kaur, A. Singh, P. Singla, Synthesis and antibacterial activity of some new benzothiazole derivatives, Acta Pharm. Sci. 52 (2010) 213-218. |
| [15] | G. Alang, R. Kaur, A. Singh, et al., Synthesis, characterization and antifungal activity of certain (E)-1-(1-(substituted phenyl)ethylidene)-2-(6-methylbenzo[ d]thiazol-2-yl)hydrazine analogues, Int. J. Pharm. Biol. Arch. 1 (2010) 56-61. |
| [16] | H.K. Barot, G. Mallika, B.B. Sutariya, J. Shukla, L.V.G. Nargund, Synthesis of nitrogen mustards of fluoro-benzothiazoles of pharmacological interest, Res. J. Pharm. Biol. Chem. Sci. 1 (1) (2010) 124-129. |
| [17] | P. Yadav, D. Chauhan, N.K. Sharma, S. Singhal, 2-Substituted hydrazino-6-fluoro- 1, 3-benzothiazole: synthesis and characterization of new novel antimicrobial agents, Int. J. ChemTech Res. 2 (2010) 1209-1213. |
| [18] | A.K. Pareek, P.E. Joseph, D.S. Seth, Novel and efficient synthesis and spectral evaluation of certain new substituted pyrazolones, Orient. J. Chem. 25 (2009) 203-206. |
| [19] | R.D. Pearson, R.T. Steigbigel, H.T. Davis, S.W. Chapmann, Method for reliable determination of minimal lethal antibiotic concentrations, Antimicrob. Agents Chemother. 18 (1980) 699-708. |
| [20] | J.M. Andrews, Determination of minimum inhibitory concentrations, J. Antimicrob. Chemother. 48 (2001) 5-16. |
| [21] | R.J. Holt, Laboratory tests of antifungal drugs, J. Clin. Pathol. 28 (1975) 767-774. |
| [22] | K.H. McClean, M.K. Winson, L. Fish, et al., Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acyl homoserine lactones, Microbiology 143 (1997) 3703-3711. |
| [23] | T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods 65 (1983) 55-63. |
| [24] | F. Denizot, R. Lang, Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability, J. Immunol. Methods 89 (1986) 271-277. |
| [25] | D. Gerlier, T. Thomasset, Use of MTT colorimetric assay to measure cell activation, J. Immunol. Methods 94 (1986) 57-63. |
| [26] | Clinical Laboratory Standard Institute (CLSI), Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, Approved standard. M38- A2, Clinical Laboratory Standard Institute, Wayne, PA, USA, 2008. |
| [27] | A.K. Bhardwaj, K. Vinothkumar, N. Rajpara, Bacterial quorum sensing inhibitors: attractive alternatives for control of infectious pathogens showing multiple drug resistance, Recent Pat. Antiinfect. Drug Discov. 8 (2013) 68-83. |
| [28] | G.A. Mohamed, S.R. Ibrahim, M.I. Shaaban, S.A. Ross, Mangostanaxanthones I and Ⅱ, new xanthones from the pericarp of Garcinia mangostana, Fitoterapia 98 (2014) 215-221. |
| [29] | O.M. Vandeputte, M. Kiendrebeogo, S. Rajaonson, et al., Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1, Appl. Environ. Microbiol. 76 (2010) 243-253. |
| [30] | C.A. Lipinski, F. Lombardo, B.W. Dominy, P.J. Feeney, Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv. Drug Deliv. Rev. 46 (2001) 3-26. |
| [31] | D.F. Veber, S.R. Johnson, H.Y. Cheng, et al., Molecular properties that influence the oral bioavailability of drug candidates, J. Med. Chem. 45 (2002) 2615-2623. |
| [32] | A. Jarrahpour, J. Fathi, M. Mimouni, et al., Petra, Osiris and Molinspiration (POM) together as a successful support in drug design: antibacterial activity and biopharmaceutical characterization of some azo Schiff bases, Med. Chem. Res. 19 (2011) 1-7. |
| [33] | A. Parvez, M. Jyotsna, M.H. Youssoufi, T. Ben Hadda, Theoretical calculations and experimental verification of the antibacterial potential of some monocyclic betalactames containing two synergetic buried antibacterial pharmacophore sites, Phosphorus Sulfur Silicon Relat. Elem. 7 (2010) 1500-1510. |
| [34] | A. Parvez, J. Meshram, V. Tiwari, et al., Pharmacophores modeling in terms of prediction of theoretical physicochemical properties and verification by experimental correlations of novel coumarin derivatives produced via Betti's protocol, Eur. J. Med. Chem. 45 (2010) 4370-4378. |
| [35] | P. Ertl, B. Rohde, P. Selzer, Fast calculation of molecular polar surface area (PSA) as a sum of fragment-based contributions and its application to the prediction of drug transport properties, J. Med. Chem. 43 (2000) 3714-3717. |
| [36] | C.A. Lipinski, F. Lombardo, B.W. Dominy, P.J. Feeney, Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv. Drug Deliv. Rev. 23 (1997) 4-25. |
| [37] | O. Ursu, A. Rayan, A. Goldblum, T. Oprea, Understanding drug-likeness, WIREs Comput. Mol. Sci. 1 (2011) 760-781. |
 2015, Vol.26
2015, Vol.26