Electro-catalyst is widely used in fuel cell [1, 2, 3],dye-sensitized solar cell [4],electro-analysis [5],and electro-synthesis [6]. However,the development of a high performance electro-catalyst is a very hard work,since the shape and size of electro-catalyst vary a lot. If we can detect the catalytic activity of each nanoparticle,it will be much easier to find the best ones.
Fortunately,single molecule/nanoparticle method has been invented in catalysis by many researches [7, 8, 9, 10, 11, 12]. Currently,there are two ways to do single molecule/nanoparticle electro-catalysis. First,Peng Chen group directly did electro-catalysis of fluorescent molecule on carbon nanotubes as shown in Fig.1a [13]. In this research,fluorescent molecule needs to take part in the reaction directly. However,a lot of electro-catalytic reactions,such as hydrogen oxidation,formic acid oxidation,oxygen reduction,etc., in real application cannot be measured. Second,Allen J. Bard group used ultra-microelectrode to isolate single nanoparticle,and then measure the electro-catalytic reactions on single nanoparticle as shown in Fig.1b [14, 15, 16, 17]. This method can study the electrocatalytic reactions on single nanoparticle without fluorescent molecule taking part in. However,the sensitivity of this method is not high enough to measure slow reactions.

|
Download:
|
| Fig. 1.Schematic of three ways to do single nanoparticle catalysis. (a) Fluorescence single molecule technology [13] with working electrode (WE), reference electrode (RE) and counter electrode (CE). Fluorescent molecule directly takes part in the catalysis. (b) Ultra-microelectrode technology [14,15,16,17]. (c) Our way based on microcell and Faraday’s Law of Electrolysis. | |
In this paper,we invented a general and highly sensitive method based on microcell and Faraday’s Law of Electrolysis to measure the single non-fluorescent molecule reaction on single nanoparticle as shown in Fig.1C. This method has the advantages from fluorescence single molecule technology and Faraday’s Law. We originally presented this innovative work at 9th Sino-US Symposium on Nanoscale Science and Technology in Tianjin,China [18].
2. Experimental 2.1. Materials and characterizationsAll commercial materials were used as received unless specified. Active carbon supported Pt catalyst (Pt/AC) was prepared by completely mixing ~3 nm Pt-nanoparticles with Vulcan XC-72 active carbon (Cabot Co.) in aqueous solution. To suppress the background noise,commercial resazurin (Sigma-R7017),was further purified by thin layer chromatography (TLC). TLC was performed on a sheet of glass slide coated with a thin layer of silica. The solvent was a mixture of CH3CN,CHCl3 and CH3OH with a ratio of 9:1:1 [19].
2.2. Total internal reflection fluorescence microscope (TIRFM)Pt/C catalyst (1 mg) was added to 1 mL aqueous solution containing 50% alcohol. The mixture was sonicated for 1 h. The sonicated solution was centrifuged at 5000 rpm for 10 min to remove big particles. The resulting mixture (5 mL) was dropped on a quartz slide and dried in air. The quartz slide was then assembled into a flow cell (100 mm (height) × 2 cm (length) × 5 mm (width)) using a cover and a double sided tape as a spacer. Two holes were drilled on the quartz slide to connect to polyethylene tubing for supplying the reactant solution driven by a syringe pump. The flow channel was flushed intensively several times to remove weakly bound Pt/C nanoparticles on the slide,before single-molecule imaging experiments were performed. Then the reactant solution containing 1-2 mmol/L resazurin and 0.5 mol/L HCOONa for the reductive N-deoxygenation reaction were injected at room temperature.
In this research,the total internal reflection fluorescence microscope (TIRFM) was performed using an Olympus IX71 Microscope. The fluorescent molecules were excited by a 532 nm laser by TIRFM. The fluorescence signal was collected by a 60X NA1.2 water-immersion objective,and detected by an ANDOR Ixon DU-897D-CS0-#BV EMCCD camera operated at 50 ms frame rate.
2.3. Epifluorescence microscopyThe excitation light with 532 nm wavelength was focused on the couple electrode through the objective lens. The fluorescence emitted by the product resorufin was focused to the detector by the same objective that is used for the excitation which for greatest sensitivity will have a very high numerical aperture 60X NA1.2.
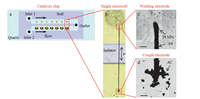
3. Results and discussion 3.1. Catalysis chip and the reactionIn order to validate the design,a catalysis chip was made as shown in Fig.2. As shown in Fig.2a,the catalysis chip have two inlets,one public outlet and many microcells. If zoom in a single microcell (Fig.2b),we can see the details of its structure. The single microcell includes one working electrode and one couple electrode,which are connected by a single Au micro-wire. A double-sided tape was used as an isolator to separate the working electrode and couple electrode. The working electrode has some Pt nanoparticles,which were immobilized by directly dropping Pt nanoparticle colloid on quartz glass. We can clearly see the drop of Pt nanoparticles in the grey area on the top of Fig.2b. If we zoom in the working electrode with Pt nanoparticles,we can see some Pt aggregates on the Au wire as shown in Fig.2c. For the couple electrode,the active carbon powder is immobilized on Au wire as shown in Fig.2d.
|
Download:
|
| Fig. 2. Images of catalysis chip. (a) Whole picture of catalysis chip. Inlet 1 is flowed into the non-fluorescent reactant, such as hydrogen, HCOONa, catalysis research. Inlet 2 is flowed into the non-fluorescent reactant, such as resazurin, for single molecule detection. The flow rate for both of these two inlets is ~10 mL/min. (b) Zoom in a single microcell. (c) Zoom in the working electrode with Pt nanoparticles. (d) Zoom in the couple electrode with active carbon for fluorescent molecule generation reaction. Scale bar is 100 mm. | |
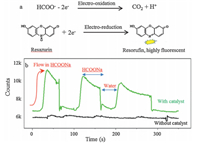
Fig.3a shows that two redox reactions,i.e. HCOONa electrooxidation reaction and resazurin electro-reduction reaction,were performed on working electrode and couple electrode,respectively. Fig.3b shows that the electrode with Pt nanoparticles had a very strong fluorescent signal,when HCCONa was flowed into the flow cell. These two reactions couple each other. But the fluorescent signal would disappear when the flow cell was washed by water. In the control experiment,the electrode without Pt nanoparticles has no signal at the same condition. So our design works very well.
|
Download:
|
| Fig. 3. Working performance of a single microcell. (a) HCOONa electro-oxidation reaction on working electrode, and fluorescent molecule generation reaction on couple electrode with active carbon. (b) Fluorescent signal (from 7 × 7 pixels) from couple electrode when formic acid oxidation reaction happens on working electrode. Green and black curves are for the electrodes with and without Pt nano-catalyst on working electrode. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) | |
In addition,Fig.3b shows some information of the stability of the Pt nano-catalyst on the working electrode. As shown in Fig.3b, the signal keeps decreasing when HCOONa was flowed into the flow cell. So the activity of Pt nano-catalyst keeps decreasing with time due to the poisoning of CO-like intermediates. This result is very similar as that from the measurement of conventional technology. This simple method is estimated to measure the current as low as 10-14 A,which means we have detected ~104 fluorescent molecules per second (or turnovers of HCOONa electro-oxidation reaction).
As we all know that the electrochemical reactions like formic acid oxidation,methanol oxidation,hydrogen oxidation and oxygen reduction are very important in real applications. But these reactions do not include any fluorescent molecule. So these reactions cannot be studied by the previous research strategy which needs the fluorescent molecule to take part into the target catalytic reaction directly. In this paper,in order to study these reactions at the single molecule/nanoparticle level,we couple these reactions with a fluorescence reaction. Then we can study the SMECNFM by measuring the fluorescence reaction on couple electrode. Here,we use resazurin reduction reaction to generate highly fluorescent resorufin. By detecting each single resorufin molecule,we can detect the formic acid electro-oxidation at single molecule level. This method is actually a general method,which can be used to measure the single non-fluorescent molecule reactions,as long as we have a fluorescence reaction with proper redox potential.
3.2. Single molecule catalysis of non-fluorescent moleculeIn principle,the above strategy is able to study the single molecule catalysis of non-fluorescent molecule on nanoparticle. However,the configuration in Fig.2 has too high noise to achieve single molecule detection. In order to improve the signal to noise ratio,we simplify the design in Figs. 1c and 2. We simply use active carbon (AC) supported Pt nanoparticle as a redox system. Since AC is not active to the electro-oxidation of non-fluorescent molecule HCOONa,but active to the electroreduction resazurin,AC could be used to detect the single molecule electro-oxidation of HCOONa on Pt nanoparticle. Therefore,the isolator and Au wire were removed from the design. The AC acts as the couple electrode,conductor and catalyst for catalytic conversion from resazurin to resorufin.
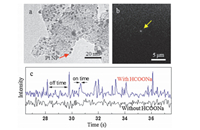
Fig.4a shows the TEM image of Pt/C catalyst with ~3 nm size. We directly immobilize Pt/C catalyst onto quartz slide to do single molecule catalysis of a non-fluorescent molecule HCOONa. Since the oxidation of HCOONa on Pt and the reduction of resazurin on active carbon do not disturb each other,the solution of HCOONa and resazurin can be feed into the microfluidic reactor together. Fig.4b shows that the product resorufin was detected when the solution including HCOONa and resazurin was flowed into the microfluidic reactor. We can see a lot of fluorescent signal from single product resorufin molecules in Fig.4c. In order to make sure that the generation of product resorufin is coupled with the oxidation of HCOONa,a control experiment without HCOONa was done. The trajectory of control experiment in Fig.4c shows that no product resorufin was detected. As a result,this research shows that we can detect the oxidation reaction of one HCOONa molecule through this method. This paper also provides a general way to study the SMECNFM for the molecules,such as formic acid,hydrogen, oxygen,etc.,on single nanoparticle.
|
Download:
|
| Fig. 4. Single molecule catalysis of non-fluorescent molecule HCOONa on Pt/AC catalyst. (a) TEM image of Pt/C catalyst. (b) Single molecule catalysis of nonfluorescent molecule in TIRFM. The yellow arrow points out the location where the reaction happens. (c) Trajectories of same location under the conditions with and without 0.5 mol/L HCOONa. The HCOONa was dissolved in pH 7.30 phosphate buffer. | |
In this paper,we use the concept of microcell in electrochemical corrosion science to study the electrochemical reaction of nonfluorescent molecule (SMECNFM) on nano-catalyst. In the microcell,the SMECNFM is coupled with a fluorescence reaction. The SMECNFM has strict stoichiometric relationship with the fluorescence reaction according to Faraday’s Law of Electrolysis. Then,the SMECNFM is detected accurately by detecting the fluorescent molecules. In this paper,we studied the oxidation reaction of one HCOONa molecule through this method. This paper also provides a general way to study the SMECNFM for the molecules,such as formic acid,hydrogen,oxygen,etc.,on single nanoparticle.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21373264 and 21573275),Suzhou Institute of Nano-tech and Nano-bionics (No. Y3AAA11004) and Thousand Youth Talents Plan (No. Y3BQA11001). The nanostructure and composition of silver nanoplates were characterized at CAS-Platform for Characterization & Test in Suzhou Institute of Nano-tech and Nano-bionics.
| [1] | X. Zhao, M. Yin, L. Ma, et al., Recent advances in catalysts for direct methanol fuel cells, Energy Environ. Sci. 4 (2011) 2736-2753. |
| [2] | Z.H. Zhou, S.L. Wang, W.J. Zhou, et al., Novel synthesis of highly active Pt/C cathode electrocatalyst for direct methanol fuel cell, Chem. Commun. (2003) 394-395. |
| [3] | Y. Zhu, Y. Kang, Z. Zou, et al., A facile preparation of carbon-supported Pd nanoparticles for electrocatalytic oxidation of formic acid, Electrochem. Commun. 10 (2008) 802-805. |
| [4] | B. O'Regan, M. Gratzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films, Nature 353 (1991) 737-740. |
| [5] | D. Chen, J. Li, Interfacial design and functionization on metal electrodes through self-assembled monolayers, Surf. Sci. Rep. 61 (2006) 445-463. |
| [6] | J.B. Sperry, D.L.Wright, The application of cathodic reductions andanodic oxidations in the synthesis of complex molecules, Chem. Soc. Rev. 35 (2006) 605-621. |
| [7] | H.P. Lu, L.Y. Xun, X.S. Xie, Single-molecule enzymatic dynamics, Science 282 (1998) 1877-1882. |
| [8] | W. Xu, J.S. Kong, Y.T.E. Yeh, P. Chen, Single-molecule nanocatalysis reveals heterogeneous reaction pathways and catalytic dynamics, Nat. Mater. 7 (2008) 992-996. |
| [9] | X. Zhou, N.M. Andoy, G. Liu, et al., Quantitative super-resolution imaging uncovers reactivity patterns on single nanocatalysts, Nat. Nano 7 (2012) 237-241. |
| [10] | T. Tachikawa, T. Majima, Single-molecule fluorescence imaging of TiO2 photocatalytic reactions, Langmuir 25 (2009) 7791-7802. |
| [11] | M.B.J. Roeffaers, B.F. Sels, H. Uji-i, et al., Spatially resolved observation of crystal-face-dependent catalysis by single turnover counting, Nature 439 (2006) 572-575. |
| [12] | W. Wang, J. Gu, T. He, et al., Optical super-resolution microscopy and its applications in nano-catalysis, Nano Res. 8 (2015) 441-455. |
| [13] | W. Xu, H. Shen, Y.J. Kim, et al., Single-molecule electrocatalysis by single-walled carbon nanotubes, Nano Lett. 9 (2009) 3968-3973. |
| [14] | X. Xiao, F.R.F. Fan, J. Zhou, A.J. Bard, Current transients in single nanoparticle collision events, J. Am. Chem. Soc. 130 (2008) 16669-16677. |
| [15] | S.J. Kwon, F.R.F. Fan, A.J. Bard, Observing iridium oxide (IrOx) single nanoparticle collisions at ultramicroelectrodes, J. Am. Chem. Soc. 132 (2010) 13165- 13167. |
| [16] | X. Xiao, A.J. Bard, Observing single nanoparticle collisions at an ultramicroelectrode by electrocatalytic amplification, J. Am. Chem. Soc. 129 (2007) 9610-9612. |
| [17] | H. Zhou, J.H. Park, F.R.F. Fan, A.J. Bard, Observation of single metal nanoparticle collisions by open circuit (mixed) potential changes at an ultramicroelectrode, J. Am. Chem. Soc. 134 (2012) 13212-13215. |
| [18] | X. Zhou, P. Chen, Application of optical super resolution imaging technology in single nanoparticle catalysis, in: 9th Sino-US Symposium on Nanoscale Science and Technology, Tianjin, 2014. |
| [19] | K.S. Han, G. Liu, X. Zhou, R.E. Medina, P. Chen, How does a single pt nanocatalyst behave in two different reactions? A single-molecule study, Nano Lett. 12 (2012) 1253-1259. |
 2015, Vol.26
2015, Vol.26 


