b State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences, Beijing 100050, China;
c State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100080, China
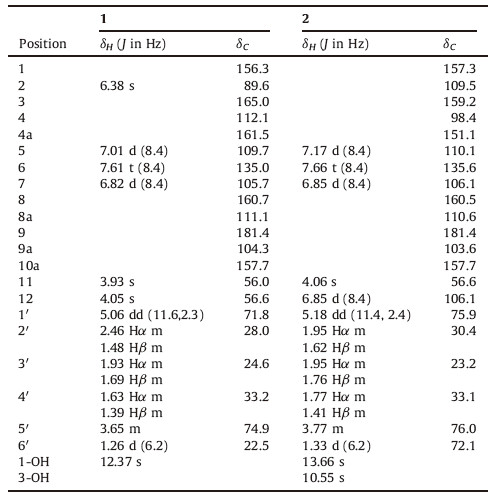
Evidence accumulated that fungi conceive species diversity, which implies their genetic diversity,and then transforming into structural diversity: secondary metabolites possessing rich and complex skeleton features with a wide range of bioactivities [1, 2]. Chaetomium sp. as one very big member of Ascomycetes,is widely distributed in soil,dung,fair,nail,paper and other rich fibrin materials around the world. Up to date,more than two hundred of secondarymetaboliteswere isolated fromthis genus [3, 4, 5, 6],whereas only a compound isocochliodinol was isolated from Chaetomium murorum [7]. During our ongoing work of mining secondary metabolites from Chaeotmium sp. [8, 9],chaetoxanthone D (1),a new tetrahydropyran-substituted xanthone originated from polyketide pathway,together with the four known natural products chaetoxanthone C (2),alternariol methyl ether (3),alternariol (4) and 2,5-dimethyl-7-hydroxychromone (5) was isolated from C. murorum (Fig. 1). In this letter,we reported planar and stereochemical establishment of 1 and 2.
|
Download:
|
| Fig. 1.Structures of compounds 1–5. | |
All 1D and 2D NMR spectra were measured on a Bruker Avance III 600 spectrometer. HRESIMS were obtained using a TOF-ESI-MS (Waters Synapt G2,USA). IR spectra were recorded on a Shimadzu FTIR-8400s. UV spectra were measured on a Shimadzu UV-2550 UV-vis spectrophotometer. CD spectra were performed on a JASCO J-810 spectrometer. Optical rotations were run on a JASCO DIP- 1000 polarimeter. Semi-preparative HPLC separation was performed on a Schimadzu LC-6AD instrument packed with a YMCPack ODS-A column (5 μm,250 × 10 mm). Sephadex LH-20 (Pharmacia Biotech AB,Uppsala,Sweden) and silica gel (200- 300 mesh) (Qingdao Marine Chemical Plant,Qingdao,China) were used for column chromatography,respectively.
2.2. Fungal materialC. murorumwas kindly provided by associate professor Xue-Wei Wang,and identified by Dr. Bin-Da Sun based on morphological features and sequence analysis of the ITS region of the rDNA (GenBank accession No. KT347318) and assigned the accession [10TD$DIF]No. CGMCC3.11382 in China General Microbiological Culture Collection Center at the Institute of Microbiology,Chinese Academy of Sciences,Beijing. The fungus C. murorum was grown on PDA plates at 25 ℃ for 7 days. Then the fresh mycelium was inoculated into autoclaving sterilized solidmediumwith the formula of rice (60.0 g) and distilled water (80 mL) in Fernbach flasks (500 mL) for further fermentation at 25 ℃ for 40 days.
2.3. Extraction and isolationThe fermented solid medium was extracted with EtOAc three times at room temperature assisted with supersonic treatment. The solvent was then evaporated under reduced pressure to afford a crude residue (3.0 g). The original extract was firstly fractionated on a silica gel column using petroleum ether-acetone (40:1-1:1) to give six fractions (Fr.1 to Fr.6). Fr.3 (120 mg) was performed on Sephadex LH-20 (MeOH-CH2Cl2) to give seven subfractions (Fr.3.1 to Fr.3.7). Fr.3.5 was further purified by semi-preparative HPLC (80% MeOH in H2O for 2 min,and followed by [1TD$DIF]80%-100% for 30 min) to yield chaetoxanthone D (1; 4.0 mg,tR 26.2 min) and Fr.3.6 was purified similarly by semi-preparative HPLC (80% MeOH in H2O for 2 min,and followed by 80%-100% for 30 min) to obtain chaetoxanthone C (2; 1.0 mg,tR 28.1 min). Fr.3.7 was further performed on Sephadex LH-20 (MeOH-CH2Cl2) to give 2,5- dimethyl-7-hydroxychromone (5,12.0 mg). Fr.4 (45 mg) and Fr.6 (80 mg) were recrystallized in Me2CO to achieve alternariol (4,10.0 mg) and alternariol methyl ether (3,8.0 mg),respectively.
2.4. Quantum-chemical calculationThe absolute configuration of compound 1/2 was established by a combination of ECD experiments and quantum-chemical calculations adopting time-dependent density functional theory (TDDFT). All quantum-chemical calculations have been performed on the (1'S,5'S,1/2a and 1'R,5'R,1/2b) configurations of 1/2 by the Gaussian 09 program package. A systematic conformational analysis was carried out using the MMFF94 force field via the MOE software package [10, 11]. The obtained conformers were further optimized and verified as the true minima of the potential energy surface at the B3LYP/6-31G(d) basis set level using the TDDFT method. The 40 lowest electronic transitions were calculated,and dipole velocity (Ravel) representations were converted to a Gaussian-type curve with a half-bandwidth of 0.4 eV to achieve the calculated ECD spectra of compound 1/2.
Chaetoxanthone D (1): Yellow amorphous powder (CH2Cl2); [α]25 D -21 (c 0.07,MeCN); UV (MeCN) λmax (log ε): 316 (0.39),245 (0.83) nm; IR υmax 3443,2926,2853,1639,1608,1482,1452,1327, 1296,1272,1244,1203,1177,1145,1123,1095,1029,992,888, 862,812,774 cm-1; CD (CH2Cl2) Δε (nm) +0.5 (246); 1H NMR (CDCl3,600 MHz),see Table 1; 13C NMR (CDCl3,150 MHz),see Table 1; (+)-HR-ESI-MS m/z 371.1503[M + H]+ (calcd. for C21H23O6, 371.1495).
| Table 1 NMR spectroscopic data for compounds 1 and 2. |
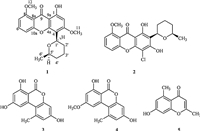
Compound 1 was isolated as a yellow amorphous powder. Its molecular formula was determined to be C21H22O6 on the basis of HR-ESI-MS (m/z 371.1503 [M + H]+; calcd. 371.1495),indicating 11 unsaturation degrees. Analysis of the 1H NMR,13C NMR, especially the HMQC data displayed that there existed 12 sp2 hybridized carbons,a ketone group with chemical shift value at 181.4 ppm,implying its connection with two double bonds,two methoxyls,four methylene units,two oxymethines,and a methyl group. The 1H NMR revealed a 1,2,3-trisubstituted phenyl with a keto group at the para-position accounting for the low chemical value of H-6 at 7.61 ppm (triplet). The 1H-1H COSY NMR data revealed two isolated proton spin-systems corresponding to the 1,2,3-trisubstituted phenyl and C-1'-C-2'-C-3'-C-4'-C-5'-C-6' , and the remaining connectivities were solved by HMBC correlations (Fig. 2). The correlations from H-5 and H-7 and to C-8a,and C- 10a,especially to C-9 (W long-ranged correlations) confirmed the 1,2,3-trisubstituted phenyl with a keto group at the para-position. The low chemical shift value of the hydroxyl group (1-OH) implied a hydrogen bond existed in 1,together with the HMBC correlations from H-2 to C-9 and C-9a confirmed the connection between C-9 and C-9a. The correlations of H-2 with C-1,C-3,C-4,and C-9a,H-1' with C-3,C-4 and C-4a suggested a five-substituted aromatic ring. The HMBC correlation from H-1' to C-5' together with 1H-1H COSY correlation established a pyran ring. The two methoxyls were anchored at C-3 and C-8,respectively,based on the HMBC correlations. Accounting for the chemical shift values C-4a and C- 10a,and degrees of unsaturation,C-4a and C-10a was connected by an oxygen atom to shape a xanthone framework. Thus the planar structure for 1 was characterized. The relative configuration was elucidated by NOESY correlations. The distinct correlations between H-10 and H-50 in the NOESY spectra confirmed these two protons on the same side of the pyran ring system.
|
Download:
|
| Fig. 2.1H–1H COSY, key HMBC and NOESY correlations of compound 1. | |
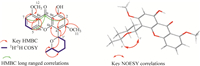
Recently,quantumchemical electronic circular dichroism (ECD) calculation as a very powerful method was used to determine the absolute configuration of natural products [12, 13]. Due to the fact that there is no suitable functional group for chemical reactions or suitable crystal for X-ray to establish the stereochemistry for compound 1,the absolute configuration of 1 was tentatively determined by using quantum chemical electronic circular dichroism (ECD) calculations,which adopted the time-dependent density functional theory (TDDFT) method. In the calculated and experimental ECD spectra of compound 1 (Fig. 3),1a represented the configuration of (10S,50S) and 1b represented its enantiomer (10R, 50R). It was clear that the calculated ECD spectrum of 1b was matched verywell with the experimental ECD spectrumof 1 (Fig. 3). Thus,the stereochemistry of 1 was determined to be 1'R,and 5'R.
|
Download:
|
| Fig. 3.The calculated ECD and experimental ECD spectra of 1 and 2. | |
Chaetoxanthone C (2) was ever isolated from a marine-derived fungus Chaetomium sp. [14]. Its planar structure was determined by NMR data,and its relative configuration was established by NOE experiment,while its stereochemistry was not characterized. Thus the absolute configuration of 2 was tried by ECDmethod. Similar to 1,2a represented the configuration of (10S,50S) and 2b represented its enantiomer (10R,50R). The calculated ECD spectrum of 2b was matched verywellwith the experimental ECDspectrumof 2 (Fig. 3). Thus,the stereochemistry of 2 was determined to be 1'R,and 5'R.
By comparing physical and spectroscopic data (MS,1H NMR) with published literatures,the three other known compounds (3- 5) were determined as alternariol methyl ether (3) [15],alternariol (4) [15] and 2,5-dimethyl-7-hydroxychromone (5) [16].
Compounds 1-5 were evaluated against several plant pathogens including Alternaria Nees,Aspergillus flavus,Cylindrocarpon, and Fusarium moniliforme without biological activities.
4. ConclusionTwo tetrahydropyran-substituted xanthones,chaetoxanthone D (1) and chaetoxanthone C (2) together with three other known compounds (3-5) were isolated from C. murorum for the first time. The absolute configurations of 1 and 2 were determined by using quantum chemical electronic circular dichroism (ECD) calculations. Our results not only enrich the chemical diversity of secondary metabolites from Chaetomiumsp.,but also provide further evidence that the absolute configurations ofnatural productswithout suitable functional groups for chemical reactions or suitable crystal for X-ray could be determined by ECD calculation methods.
AcknowledgmentsWe gratefully acknowledge financial support from Program for Innovative Research Team in IMPLAD (PIRTI),the Open Funding Project of the State Key Laboratory of Bioactive Substance and Function of Natural Medicines,PUMC Youth Fund and the Fundamental Research Funds for the Central Universities,and the Chinese National S&T Special Project on Major New Drug Innovation (Nos. 2013ZX09508104,2011ZX09307-002-01).
Appendix A. Supplementary dataSupplementarymaterial related to this article canbe found,inthe online version,at http://dx.doi.org/10.1016/j.cclet.2015.10.025.
| [1] | R.X. Tan, W.X. Zou, Endophytes: a rich source of functional metabolites, Nat. Prod. Rep. 18 (2001) 448-459. |
| [2] | A. Schueffler, T. Anke, Fungal natural products in research and development, Nat. Prod. Rep. 31 (2014) 1425-1448. |
| [3] | Q. Zhang, H.Q. Li, S.C. Zong, et al., Chemical and bioactive diversities of the genus Chaetomium secondary metabolites, Mini-Rev. Med. Chem. 12 (2012) 127-148. |
| [4] | L.M. Li, Q. Zou, G.Y. Li, Chromones from an ascomycete, Chaetomium aureus, Chin. Chem. Lett. 21 (2010) 1203-1205. |
| [5] | J.M. Gao, S.X. Yang, J.C. Qin, Azaphilones: chemistry and biology, Chem. Rev. 113 (2013) 4755-4811. |
| [6] | H. Li, J.M. Tian, H.Y. Tang, et al., Chaetosemins A-E, new chromones isolated from an ascomycete Chaetomium seminudum and their biological activities, RSC Adv. 5 (2015) 29185-29192. |
| [7] | S. Setsuko, Isocochliodinol and neocochliodinol, bis(3-indolyl)-benzoquinones from Chaetomium spp, Chem. Pharm. Bull. 31 (1983) 2998-3001. |
| [8] | H.M. Ge, W.Y. Zhang, G. Ding, et al., Chaetoglobins A and B, two unusual alkaloids from endophytic Chaetomium globosum culture, Chem. Commun. 45 (2008) 5978-5980. |
| [9] | G. Ding, Y.C. Song, J.R. Chen, et al., Chaetoglobosin U, a cytochalasan alkaloids from endophytic Chaetomium globosum IFB-E019, J. Nat. Prod. 69 (2006) 302-304. |
| [10] | Gaussian, Inc, Gaussian 09, Revision B. 01, Gaussian, Inc, Wallingford, CT, 2009. |
| [11] | Molecular Operating Environment (MOE), Chemical Computing Group Inc., Montreal, Canada, 2015 (http://www.chemcomp.com/Research-Citing_MOE.htm). |
| [12] | G. Ding, H.L. Wang, Li Li, et al., Trichodermone, a spiro-cytochalasan with a tetracyclic nucleus (7/5/6/5) skeleton from the plant endophytic fungus Trichoderma gamsii, J. Nat. Prod. 77 (2014) 164-167. |
| [13] | G. Ding, H.L. Wang, Li Li, et al., Trichoderones A and B: two pentacyclic cytochalasans from the plant endophytic fungus Trichoderma gamsii, Eur. J. Org. Chem. 2012 (2012) 2516-2519. |
| [14] | P. Alexander, K. Anja, K. Stefan, et al., Antiprotozoal activities of heterocyclicsubstituted xanthones from the marine-derived fungus Chaetomium sp, J. Nat. Prod. 71 (2008) 1579-1584. |
| [15] | K. Koch, J. Podlech, E. Pfeiffer, et al., Total synthesis of alternariol, J. Org. Chem. 70 (2005) 3275-3276. |
| [16] | S. Kametani, A. Kojima, H. Kikuzaki, et al., Chemical constituents of cape aloe and their synergistic growth-inhibiting effect on ehrlich ascites tumor cells, Biosci. Biotechnol. Biochem. 71 (2007) 1220-1229. |
 2015, Vol.26
2015, Vol.26