b Shanghai Collaborative Innovation Center for Biomanufacturing Technology, Shanghai 200237, China
Root-knot nematodes of the Meloidogyne genus represent a serious and growing threat to agriculture [1],causing an estimated annual crop loss of more than $100 billion worldwide [2]. As the most damaging Meloidogyne species throughout the world [3, 4], Meloidogyne incognita is a sedentary endoparasitic phytonematode attacking the roots of various crops and usually facilitating the secondary infection of other soilborne phytopathogens to crops [5- 9]. Thus the management of root-knot nematodes has become an important task in front of us. Nowadays,synthetic nematicides are the most commonly used weapon for phytonematode control [2, 10],despite that some of them such as methyl bromide, fenamiphos and aldicarb have been restricted or withdrawn from the market due to their adverse impacts on human health and environment [11, 12, 13]. Compared with insecticides,herbicides and fungicides,nematicides have a relatively small market and only a few nematicides are applicable [14, 15],which makes it very difficult for farmers to fight against nematodes. Therefore,new chemotypes of nematicides with high nematicidal activity and low toxicity to non-target organisms are urgently needed to ensure the sustainable development of modern agriculture.
1,2,3-Benzotriazin-4-one is a commonly usedmotif in a variety of pharmaceutical and agrochemical compounds [16, 17, 18, 19, 20, 21]. For example,azinphos-methyl (APM) and azinphos-ethyl (APE) are widely used broad-spectrum insecticides and acaricides in crop protection [22]. The first reported nematicidal 1,2,3-benzotriazin- 4-one derivative (Fig. 1A)was active against Anguillula nematodes [21]. After that,1,2,3-benzotriazin-4-ones were rarely reported in the field of nematicide. Until recently,we have reported that 1,2,3-benzotriazin-4-one derivatives bearing 2-cyanoiminothiazolidin- 4-one moieties (Fig. 1B) had significant inhibitory activities against M. incognita,which can be utilized as lead compounds to study further [23]. In addition,another reported spirocyclic indoline-2-one derivative (Fig. 1C) by our research group,[3TD$DIF]possesing excellent nematicidal activity against M. incognita [24],has also attracted our considerable attention. Based on the combination of the above strucutres,we herein introduced spirocyclic indoline-2-one moieties into the 1,2,3- benzotriazin-4-one skeleton to obtain novel compounds with higher nematicidal activities (Fig. 1).
In this study,we reported the design,synthesis and nematicidal activities of 1,2,3-benzotriazin-4-one derivatives containing spirocyclic indoline-2-one moieties and their preliminary structureactivity relationship,which could also provide the basis for further structural optimization.
2. Experimental 2.1. ChemistryAll reagents and solvents were purchased from commercial suppliers without further purification. Melting points (mp) were determined on a Bu-chi Melting Point B540 instrument and are uncorrected. 1H NMR,13C NMR,and 19F NMR spectra were recorded at 400 MHz,100 MHz and 376 MHz on a Bruker AM-400 spectrometer,respectively,using DMSO-d6 as solvent. Chemical shifts are reported in parts per million (ppm,δ scale) with tetramethylsilane (TMS) as the internal standard. Coupling constants (J) are reported in hertz. High-resolution mass spectra (HRMS) were recorded under an electro-spray ionization condition on a Waters Micromass LC-TOF spectrometer. Analytical thin-layer chromatography (TLC) was performed on precoated glass plates (silica gel 60 F254),visualizing the spots by a UV lamp (254 nm).
2.2. General procedures for preparation of intermediates 2a-h,3a-h,and 4a-h are detailed in the Supporting information 2.2.1. General procedure for preparation of intermediate 5Amixture of indole-2,3-dione (isatin,5.88 g,40mmol),ethylene glycol (44 mL) and p-toluenesulfonic acid (TsOH,0.76 g,4mmol) in toluene (300 mL) was stirred and heated to reflux for 10 h. The reactionmixturewas evaporated under reduced pressure to remove the solvent. Then saturated sodium bicarbonate aqueous solution was added to the residue,which was extracted with CH2Cl2 (80 mL × 3). The combined organic layer was dried over anhydrous Na2SO4,concentrated and purified by flash columnchromatography on silica gel using petroleumether (60-90 ℃)/EtOAc (2:1) as eluent to yield 5 as light yellowsolid. Yield,83%; 1H NMR (400 MHz,DMSOd6): δ 10.43(s,1H),7.37-7.28(m,2H),7.01(td,J1 = 7.6 Hz,J2 = 0.8 Hz, 1H),6.83 (d,J = 7.6Hz,1H),4.40-4.30 (m,2H),4.30-4.20(m,2H); 13C NMR (100 MHz,DMSO-d6) δ 174.4,142.8,131.6,124.9,124.6,122.3, 110.5,101.6,65.4.
2.2.2. General procedure for preparation of title compounds 6a-pTo a solution of 5 (0.382 g,2 mmol) in DMF (15 mL),3 or 4 (2.2 mmol) and potassium carbonate (0.414 g,3 mmol) were added. The reaction mixture was heated to 80 ℃ and monitored by TLC. After the complete consumption of 5,the solvent was removed under reduced pressure. Then the residue was taken up in water and extracted with CH2Cl2 (30 mL × 3). The combined organic layer was dried over anhydrous Na2SO4,concentrated and purified by flash column chromatography on silica gel using petroleum ether (60-90 ℃)/EtOAc (3:1) as eluent to yield 6. The data of compounds 6a-h are listed as follows,and other data of 6i- p are deposited in the Supporting Information.
1-(3-(4-Oxobenzo[d][1, 2, 3]triazin-3(4H)-yl)propyl)spiro[indoline- 3,20-[1, 3]dioxolan]-2-one (6a): yield,60%; mp 174.9-175.3 ℃; 1H NMR (400 MHz,DMSO-d6): δ 8.25 (d,J = 8.0Hz,1H),8.20 (d, J = 8.0 Hz,1H),8.09 (t,J = 7.6 Hz,1H),7.93 (t,J = 8.0Hz,1H),7.43 (t, J = 7.6Hz,1H),7.39 (d,J = 7.6Hz,1H),7.17 (d,J = 7.6Hz,1H),7.11 (t, J = 7.6Hz,1H),4.43 (t,J = 7.2 Hz,2H),4.39-4.23 (m,4H),3.78 (t, J = 7.2Hz,2H),2.28-2.09 (m,2H); 13C NMR (100 MHz,DMSO-d6): δ 172.5,154.8,143.7,143.3,135.3,132.8,131.7,127.9,124.7,124.5, 124.1,123.0,119.3,109.5,101.4,65.5,47.0,36.8,26.2. HRMS (ES+) calcd. for C20H18N4O4Na (M + Na)+,401.1226,found,401.1222.
1-(3-(4-Oxo-6-methylbenzo[d][1, 2, 3]triazin-3(4H)-yl)propyl)- spiro[indoline-3,20-[1, 3]dioxolan]-2-one (6b): yield,38%; mp 142.0-142.6 ℃; 1H NMR (400 MHz,DMSO-d6): d 8.09 (d, J = 8.4 Hz,1H),8.04 (s,1H),7.90 (dd,J1 = 8.4 Hz,J2 = 1.2 Hz,1H), 7.43 (t,J = 8.0 Hz,1H),7.39 (d,J = 7.2 Hz,1H),7.16 (d,J = 7.6 Hz, 1H),7.10 (t,J = 7.6 Hz,1H),4.41 (t,J = 7.2 Hz,2H),4.38-4.24 (m, 4H),3.77 (t,J = 7.2 Hz,2H),2.55 (s,3H),2.21-2.09 (m,2H); 13C NMR (100 MHz,DMSO-d6): δ 172.5,154.8,143.7,143.3,142.0,136.4, 131.7,127.9,124.7,124.1,123.7,123.0,119.1,109.5,101.4,65.5, 46.9,36.8,26.3,21.3. HRMS (ES+) calcd. for C21H20N4O4Na (M + Na)+,415.1382,found,415.1384.
1-(3-(4-Oxo-7-methoxylbenzo[d][1, 2, 3]triazin-3(4H)-yl)propyl) spiro[indoline-3,20-[1, 3]dioxolan-2-one (6c): yield,27%; mp 135.1-135.6 ℃; 1H NMR (400 MHz,DMSO-d6): δ 8.13 (d,J = 8.8 Hz, 1H),7.61 (d,J = 1.6 Hz,1H),7.46 (dd,J1 = 8.8 Hz,J2 = 2.0 Hz,1H), 7.42 (d,J = 8.0 Hz,1H),7.39 (d,J = 7.2 Hz,1H),7.15 (d,J = 8.0 Hz, 1H),7.10 (t,J = 7.6 Hz,1H),4.40 (t,J = 7.2 Hz,2H),4.37-4.25 (m, 4H),3.98 (s,3H),3.77 (t,J = 7.2 Hz,2H),2.20-2.08 (m,2H); 13C NMR (100 MHz,DMSO-d6): δ 172.5,164.3,154.4,145.9,143.3,131.7, 126.3,124.7,124.1,123.0,122.3,112.7,109.5,108.6,101.4,65.5, 56.3,46.8,36.8,26.3. HRMS (ES+) calcd. for C21H20N4O5Na (M + Na)+,431.1331,found,431.1328.
1-(3-(4-Oxo-7-bromobenzo[d][1, 2, 3]triazin-3(4H)-yl)propyl)- spiro[indoline-3,20-[1, 3]dioxolan]-2-one (6d): yield,54%; mp 165.2-165.6 ℃; 1H NMR (400 MHz,DMSO-d6): δ 8.46 (d, J = 2.0 Hz,1H),8.14 (d,J = 8.4 Hz,1H),8.07 (dd,J1 = 8.4 Hz, J2 = 1.6 Hz,1H),7.43 (td,J1 = 8.0 Hz,J2 = 1.2 Hz,1H),7.38 (d, J = 7.2 Hz,1H),7.16 (d,J = 8.0 Hz,1H),7.10 (t,J = 7.6 Hz,1H),4.41 (t, J = 7.2 Hz,2H),4.37-4.24 (m,4H),3.78 (t,J = 7.2 Hz,2H),2.21-2.11 (m,2H); 13C NMR (100 MHz,DMSO-d6): δ 172.5,154.4,144.5, 143.3,135.7,131.7,130.2,128.6,126.8,124.7,124.0,123.0,118.5, 109.5,101.4,65.5,47.2,36.8,26.1. HRMS (ES + ) calcd. for C20H17N4O4Na79Br (M + Na)+,479.0331,found,479.0336; calcd. for C20H17N4O4Na81Br (M + Na)+,481.0310,found,481.0304.
1-(3-(4-Oxo-7-chlorobenzo[d][1, 2, 3]triazin-3(4H)-yl)propyl)- spiro[indoline-3,20-[1, 3]dioxolan]-2-one (6e): yield,29%; mp 172.2-172.7 ℃; 1H NMR (400 MHz,DMSO-d6): d 8.32 (s,1H), 8.23 (d,J = 8.4 Hz,1H),7.95 (d,J = 7.6 Hz,1H),7.43 (t,J = 7.6 Hz, 1H),7.38 (d,J = 7.2 Hz,1H),7.16 (d,J = 7.6 Hz,1H),7.10 (t, J = 7.6 Hz,1H),4.42 (t,J = 7.2 Hz,2H),4.37-4.24 (m,4H),3.78 (t, J = 6.8 Hz,2H),2.22-2.11 (m,2H); 13C NMR (100 MHz,DMSO-d6): δ 172.5,154.3,144.5,143.2,139.7,132.9,131.7,127.1,126.8,124.7, 124.0,123.0,118.2,109.5,101.4,65.5,47.1,36.8,26.1. HRMS (ES+) calcd. for C20H17N4O4Na35Cl (M + Na)+,435.0836,found,435.0841; calcd. for C20H17N4O4Na37Cl (M + Na)+,437.0807,found,437.0807.
1-(3-(4-Oxo-7-fluorobenzo[d][1, 2, 3]triazin-3(4H)-yl)propyl)- spiro[indoline-3,20-[1, 3]dioxolan]-2-one (6f): yield,30%; mp 180.3-180.8 ℃; 1H NMR (400 MHz,DMSO-d6): δ 8.31 (dd, J1 = 8.8 Hz,J2 = 6.0 Hz,1H),8.07 (dd,J1 = 8.8 Hz,J2 = 2.4 Hz,1H), 7.80 (td,J1 = 8.8 Hz,J2 = 2.8 Hz,1H),7.43 (t,J = 7.6 Hz,1H),7.38 (d, J = 7.2 Hz,1H),7.16 (d,J = 7.6 Hz,1H),7.10 (t,J = 7.6 Hz,1H),4.45- 4.39 (m,2H),4.37-4.24 (m,4H),3.78 (t,J = 7.2 Hz,2H),2.23-2.10 (m,2H); 19F NMR (376 MHz,DMSO-d6): δ -102.0 (td,J1 = 8.6 Hz, J2 = 5.6 Hz). HRMS (ES+) calcd. for C20H17N4O4FNa (M + Na)+, 419.1132,found,419.1135.
1-(3-(4-Oxo-6-(trifluoromethyl)benzo[d][1, 2, 3]triazin-3(4H)- yl)propyl)spiro[indoline-3,20-[1, 3]dioxolan]-2-one (6g): yield, 78%; mp 163.1-163.4 ℃; 1H NMR (400 MHz,DMSO-d6): δ 8.47 (s,1H),8.44-8.37 (m,2H),7.43 (t,J = 7.6 Hz,1H),7.37 (d,J = 7.2 Hz, 1H),7.16 (d,J = 8.0 Hz,1H),7.09 (t,J = 7.6 Hz,1H),4.46 (t,J = 7.2 Hz, 2H),4.36-4.23 (m,4H),3.79 (t,J = 6.8 Hz,2H),2.26-2.13 (m,2H); 19F NMR (376 MHz,DMSO-d6): δ -61.5 (s). HRMS (ES+) calcd. for C21H17N4O4F3Na (M + Na)+,469.1100,found,469.1100.
1-(3-(4-Oxo-7-nitrobenzo[d][1, 2, 3]triazin-3(4H)-yl)propyl)- spiro[indoline-3,20-[1, 3]dioxolan]-2-one (6h): yield,36%; mp 154.5-154.9 ℃; 1H NMR (400 MHz,DMSO-d6): δ 8.91 (d, J = 2.0 Hz,1H),8.59 (dd,J1 = 8.4 Hz,J2 = 2.0 Hz,1H),8.45 (d, J = 8.8 Hz,1H),7.43 (td,J1 = 7.6 Hz,J2 = 0.8 Hz,1H),7.37 (d, J = 7.2 Hz,1H),7.16 (d,J = 8.0 Hz,1H),7.09 (t,J = 7.2 Hz,1H), 4.45 (t,J = 7.2 Hz,2H),4.36-4.23 (m,4H),3.79 (t,J = 7.2 Hz,2H), 2.25-2.14 (m,2H); 13C NMR (100 MHz,DMSO-d6): δ 172.5,153.9, 151.3,143.7,143.2,131.7,127.2,126.2,124.7,124.0,123.5,123.2, 123.0,109.5,101.3,65.5,47.4,36.8,25.9. HRMS (ES+) calcd. for C20H17N5O6Na (M + Na)+,446.1077,found,446.1071.
2.3. Nematicidal evaluation [23]Pure compounds (6a-p) were dissolved with DMF and diluted with distilled water to obtain series concentrations (10.0,5.0,and 1.0 mg/L) for bioassays. The final concentration of DMF in each treatment never exceeded 1% v/v. The one-week age cucumber seedlings were planted in sterilized sand in test tubes (one seedling per test tube,tube size: 20 × 250 mm),and treated the roots of each plant with 3 mL of test solution. Then approximately 2000 living second-stage juveniles (J2) of M. incognita were inoculated into the rhizosphere sand of each plant. Avermectin (B1) at 5.0 and 1.0 mg/L served as positive control,and the negative control group was prepared in the same way but lacked the tested compound. All the test tubes were incubated at 20-25 ℃ for 20 days,with 10 h in the daylight per day. The number of root knots of each plant was counted and recorded a score. The inhibition rate on M. incognita was calculated by comparison with the negative control group:

Scoring criteria: 0: 0-5 knots; 5: 6-10 knots; 10: 11-20 knots; 20: more than 20 knots.
3. Results and discussion 3.1. SynthesisAsdepicted in Scheme1,intermediates 2a-h were preparedfrom anthranilamides 1a-h via diazotization,nucleophilic addition and cyclization reactions in one pot [25],in which electron donation (CH3,OCH3) and electron withdrawal (F,CF3,NO2,etc.) were well tolerated. Then 2a-h were alkylated at N-3 position under the weakly basic (K2CO3) condition to afford intermediates 3a-h and 4a-h,using 1,3-dibromopropane and 1,4-dibromobutane as alkylation agents,respectively [26]. It was noteworthy that more than 5 equiv. of dibromoalkane was necessary to minimize the biscoupling product. In the N-alkylation of compound 2,N-3 alkylated ones were the main products. Miranda et al. predicted 1,2,3- benzotriazin-4(3H)-one to be the most stable of its three tautomers [27]. To confirm the structures of N-3 alkylated products,2D NMR (HMBC) was performed. Fromthe HMBC spectrum(500MHz) of 3- (3-bromopropyl)-7-chlorobenzo[d][1, 2, 3]triazin-4(3H)-one,it can be seen that the methylene (H-5) is strongly related to the carbonyl group (C-4) of benzotriazinone but not related to the benzene ring (The corresponding spectra are deposited in the Supporting Information). The spirocyclic indoline-2-one intermediate 5 was synthesized by the reaction of indole-2,3-dione and ethylene glycol with 10 mol% of TsOH as catalyst. Finally,in the presence of potassiumiodide,intermediates 3a-h or 4a-h reactedwith5inDMF at 80 ℃ to afford title compounds 6a-p. The structures of all title compoundswere confirmed by 1 [1TD$DIF]HNMR,13CNMR,and 19F NMR and HRMS (ESI-TOF).
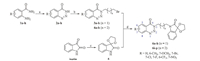
|
Download:
|
| Scheme. 1.Synthetic procedure for title compounds 6a–p. Reagents and conditions: (a) i. NaNO2, 0.5 M HCl, DMF-H2O, 0 ℃, 1 h; ii. NH3·H2O, pH = 8–10, 15 min; (b) dibromoalkane, K2CO3, acetone, reflux, 5–8 h; (c) ethylene glycol, TsOH, toluene, reflux, 10 h; (d) KI, DMF, 80 ℃, 10–15 h. | |
The nematicidal activities of all title compounds were evaluated against root-knot nematodes (M. incognita) in vivo and the corresponding data are shown in Table 1. Avermectin was also tested as an assay positive control to provide a benchmark of activity.
| Table 1 Nematicidal activities of compounds 6a–p against M. incognita in test tubes. |
At the treatment concentration of 10.0 mg/L,most compounds showed >50% inhibitory activities against M. incognita. (a) When the linker was fixed as a three-carbon chain,compound 6a had the most potent inhibitory effect on nematodes with the inhibition rate of 85.6%,followed by compound 6ewith Cl group. It seemed that electron donation (CH3,OCH3) or electron withdrawal (F,CF3, NO2,[1TD$DIF]etc.) had no obvious influence on inhibitory activity. For electron-donating groups substituted compounds,compound 6c (OCH3) had higher activity than compound 6b (CH3). For electronwithdrawing groups substituted compounds,compound 6e (Cl) was the most potent. (b) However,when the length of linker was extended to four carbons,the activities of compounds 6i-p did not follow the similar regularity with those of compounds 6a-h with a three-carbon chain. Compared to the unsubstituted compound 6i, the introduction of strong electron-donating OCH3 or electronwithdrawing NO2 into the benzotriazinone ring significantly increased the inhibitory activity,up to 100% at 10.0 mg/L (e.g., 6k,6p). (c) For the same substituents at the same positions on the benzotriazinone ring,unsubstituted or OCH3,Br,NO2 substituted compounds with a four-carbon chain had high inhibitory potency than that of compounds with a shorter linker (e.g.,6i vs 6a; 6k vs 6c; 6l vs 6d; 6p vs 6h),while CH3,Cl,F,or CF3 substituted compounds displayed an opposite regularity (e.g.,6j vs6b; 6m vs 6e; 6n vs 6f; 6o vs 6g). From all the results above,it can be concluded that the nematicidal activity was influenced by the coeffect of substituent and linker length.
Among all the synthesized compounds,only compounds 6k and 6p exhibited 100% inhibitory activities against M. incognita,which was the most promising for further structural optimization. Then the lower treatment concentrations of these two compounds at 5.0 and 1.0 mg/L were tried. It was found that the inhibitory activities decreased dramatically with only <35% inhibition rates. Compared to the reported spirocyclic indoline-2-one derivative C (Fig. 1) with the inhibition rate of 28.1% at 5.0 mg/L [24],the inhibitory activity of compound 6p was a bit higher. Further structural optimization is now still under way.

|
Download:
|
| Fig. 1.Design strategy of title compounds (6a–p). | |
In summary,a series of novel 1,2,3-benzotriazin-4-one derivatives containing spirocyclic indoline-2-one moieties were synthesized. The nematicidal assays showed that most of the title compounds had moderate to good nematicidal activities against M. incognita at 10.0 mg/L. In particular,compounds 6k and 6p were the most potent with 100% inhibition rates,which can be regarded as lead compounds for further structural modification to find new potential nematicides.
AcknowledgmentsThis work was financial supported by the National High Technology Research and Development Program of China (National 863 Program,No. 2013AA065202),and the National Natural Science Foundation of China (No. 21272071). This work was also partly supported by the Special Fund for Agro-scientific Research in the Public Interest (No. 201103007) and the Fundamental Research Funds for the Central Universities.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.10.024.
| [1] | N.M. Truong, C.N. Nguyen, P. Abad, et al., Chapter Twelve—Function of root-knot nematode effectors and their targets in plantaparasitism, Adv. Bot. Res. 73 (2015) 293-324. |
| [2] | J.P. McCarter, Molecular approaches toward resistance to plant parasitic nematodes, in: R.H. Berg, C.G. Taylor (Eds.), Cell Biology of Plant Nematode Parasitism, Springer-Verlag Press, Berlin, 2009, pp. 83-113. |
| [3] | J.N. Sasser, Worldwide dissemination and importance of the root-knot nematodes Meloidogyne spp, J. Nematol. 9 (1977) 26-29. |
| [4] | R.O. Rocha, J.K.S. Morais, J.T.A. Oliveira, et al., Proteome of soybean seed exudates contains plant defense-related proteins active against the root-knot nematode Meloidogyne incognita, J. Agric. Food Chem. 63 (2015) 5335-5343. |
| [5] | D.L. Trudgill, V.C. Blok, Apomictic polyphagous root-knot nematodes exceptionally successful and damaging biotrophic root pathogens, Ann. Rev. Phytopathol. 39 (2001) 53-77. |
| [6] | M.A. Back, P.P.J. Haydock, P. Jenkinson, Disease complexes involving plant parasitic nematodes and soilborne pathogens, Plant Pathol. 51 (2002) 683-697. |
| [7] | P. Caboni, M. Saba, G. Tocco, et al., Nematicidal activity of mint aqueous extracts against the root-knot nematode Meloidogyne incognita, J. Agric. Food Chem. 61 (2013) 9784-9788. |
| [8] | P. Caboni, N. Aissani, M. Demurtas, et al., Nematicidal activity of acetophenones and chalcones against Meloidogyne incognita and structure-activity considerations, Pest Manage. Sci. (2015), http://dx.doi.org/10.1002/ps.3978. |
| [9] | Y. Huang, L. Ma, D.H. Fang, et al., Isolation and characterisation of rhizosphere bacteria active against Meloidogyne incognita, Phytophthora nicotianae and the root knot-black shank complex in tobacco, Pest Manage. Sci. 71 (2015) 415-422. |
| [10] | P.P.J. Haydock, S.R. Woods, I.G. Grove, et al., Chemical control of nematodes, in: R.N. Perry, M. Moens (Eds.), Plant Nematology, CABI, Wallingford, CT, 2006, pp. 392-408. |
| [11] | V.L. Fuller, C.J. Lilley, P.E. Urwin,Nematode resistance,NewPhytol.180 (2008) 27-44. |
| [12] | S. Qin, J. Gan, W. Liu, et al., Degradation and absorption of fosthiazate in soil, J. Agric. Food Chem. 52 (2004) 6239-6242. |
| [13] | Y. Hu, W. Zhang, P. Zhang, et al., Nematicidal activity of chaetoglobosin A produced by Chaetomium globosum NK102 against Meloidogyne incognita, J. Agric. Food Chem. 61 (2013) 41-46. |
| [14] | P. Caboni, L. Tronci, B. Liori, et al., Tulipaline A: structure-activity aspects as a nematicide and V-ATPase inhibitor, Pest. Biochem. Physiol. 112 (2014) 33-39. |
| [15] | Z. Che, S. Zhang, Y. Shao, et al., Synthesis and quantitative structure-activity relationship (QSAR) study of novel N-arylsulfonyl-3-acylindole arylcarbonyl hydrazone derivatives as nematicidal agents, J. Agric. Food Chem. 61 (2013) 5696-5705. |
| [16] | G. Caliendo, F. Fiorino, P. Grieco, et al., Preparation and local anaesthetic activity of benzotriazinone and benzoyltriazole derivatives, Eur. J. Med. Chem. 34 (1999) 1043-1051. |
| [17] | M.J. Kornet, Microwave synthesis and anticonvulsant activity of new 3-benzyl- 1,2,3-benzotriazin- 4(3H)-ones, J. Heterocyclic Chem. 34 (1997) 1391-1393. |
| [18] | T.S. Ibrahim, A.A. Rashad, Z.K. Abdel-Samii, et al., Synthesis, molecular modeling and anti-inflammatory screening of new 1,2,3-benzotriazinone derivatives, Med. Chem. Res. 21 (2012) 4369-4380. |
| [19] | D. Raffa, G. Daidone, B. Maggio, et al., Synthesis and antiproliferative activity of novel 3-(indazol-3-yl)-quinazolin-4(3H)-one and 3-(indazol-3-yl)-benzotriazin- 4(3H)-one derivatives, Arch. Pharm. 332 (1999) 317-320. |
| [20] | R.H. Rigterink, Insecticidal use of S-((4-oxo-1,2,3-benzotriazin-3(4H)-yl)-methyl) phosphorothioates and phosphorodithioates, US Patent 3551562, 1970. |
| [21] | J.F. Hosler, W.B. Hardy, Nu-Trichloromethylthio-1,2,3-benzotriazine-4-one as a novel nematocide, US Patent 2935445, 1960. |
| [22] | B. Liu, Y. Ge, Y. Zhang, et al., Development of a simplified enhanced chemiluminescence enzyme linked immunosorbent assay (ECL-ELISA) for the detection of phosmet, azinphos-methyl and azinphos-ethyl residues in vegetable samples, Anal. Methods 5 (2013) 5938-5943. |
| [23] | G. Wang, X. Chen, Y. Deng, et al., Synthesis and nematicidal activities of 1,2,3- benzotriazin-4-one derivatives against Meloidogyne incognita, J. Agric. Food Chem. 63 (2015) 6883-6889. |
| [24] | M. Zou, X. Tian, N. Chen, et al., Nematicidal activity of sprio and bridged heterocyclic neonicotinoid analogues against Meloidogyne incognita, Lett. Drug Des. Discovery 12 (2015) 439-445. |
| [25] | A.S. Clark, B. Deans, M.F.G. Stevens, et al., Antitumor imidazotetrazines. 32.1. Synthesis of novel imidazotetrazinones and related bicyclic heterocycles to probe the mode of action of the antitumor drug temozolomide, J. Med. Chem. 38 (1995) 1493-1504. |
| [26] | P.K.S. Sarma, S. Sharma, S. Dharmarajan, et al., Adrenergic receptor antagonists, WO Patent 2006117760, 2006. |
| [27] | M.S. Miranda, M.A.R. Matos, V.M. Morais, et al., Study of energetics and structure of 1,2,3-benzotriazin-4(3H)-one and its 1H and enol tautomers, J. Phys. Chem. B 115 (2011) 6616-6622. |
 2015, Vol.26
2015, Vol.26 



