b School of Chemical Engineering and Technology, Harbin Institute of Technology, Harbin 150001, China
In recent years,transition metal oxides-based nanomaterials have shown excellent electrochemical properties leading to a number of interesting applications in electro-catalysis,electroanalysis, and energy-related materials [1, 2, 3]. Abundant studies have shown that charge transfer via hopping processes between cations of different valencies requires relatively low activation energy. Thus,intrinsic mixed-valence systems can significantly improve the conductivity and catalytic activity of metal oxides [4]. As is well-known,spinel compounds of MFe2O4 (M = Co,Ni,Cu, etc.) are built around a closely packed array of O2- ions,with M2+ and Fe3+ cations occupying part or all of the tetrahedral and octahedral sites,respectively. More importantly,in this structure the solid-state redox couples Fe3+/Fe2+ and M3+/M2+ are widely present,which may provide a notable electro-catalytic activity in some electrochemical catalytic reactions. Usually,MFe2O4 has a common inverse spinel structure in which O forms the facecentered cubic (fcc) packing with M(II) occupying the octahedral (O) interstitial sites and Fe(III) distributing evenly in the O and tetrahedral (T) sites. Such a structure has shown good electrical conductivity due to the electron hopping between different valence states of metals in O-sites and should also provide necessary surface redox active metal centers for the adsorption and activation of electro-active species [5].
However,the ferrites NPs are prone to aggregation in the electrochemical redox processes,which will greatly decrease the exposed active sites for electro-catalysis and then result in relatively lowutilization of activematerials and inferior structure stability. Thus,tuning the shapes and structures of the spinel-type ferrites is one of the important research areas in both electrocatalysis and electro-analysis [6, 7, 8, 9]. Since the first exploration by Formhals,electrospinning,an industry-viable and versatile technique,has attracted considerable attention for producing long continuous nanofibers (NFs) with diameters ranging from several micrometers down to several nanometers [10, 11].With a post calcination treatment,it provides a straightforward and lowcost fabrication route to prepare 1D metal oxide NFs. More importantly,the as-prepared 3D net-like metal oxide films possess many exciting characteristics,including large surface area to volume ratio,good mechanical strength,excellent flexibility,high porosity,and desirable electrical conductivity [2, 12],all of which render such films promising electro-active materials.
Equipped with this knowledge,we prepared the CoFe2O4 and NiFe2O4 NFs by electrospinning and the subsequent thermal treatment processes in air. Hydrazine findswidespread applications in rocket fuels,missile systems,weapons of mass destruction,fuel cells,and corrosive inhibitors. However,hydrazine is a neurotoxin and shows carcinogenic and mutagenic effects [13, 14]. Due to the non-eco-friendliness and toxicological nature of hydrazine compounds, the development of sensitive and selective analytical methods for the detection of hydrazine is necessary. In this paper, the as-prepared CoFe2O4 and NiFe2O4 NFs are used in the selective detection of hydrazine with good sensitivity.
2. Experimental 2.1. Reagents and apparatusPolyvinylpyrrolidone (PVP,K90),N,N-dimethylformamide (DMF),were obtained from Alfa Aesar. Hydrazine,NaOH, Ni(NO3)2·6H2O,Co(NO3)2·6H2O (99.0%),and Fe(NO3)3·9H2O were purchased from Sinopharm Chemical Reagent C. All other reagents were of analytical grade and used as received. Highly purified nitrogen (≥ 99.99%) was supplied by Changchun Juyang Co.,Ltd. Ultrapure water (resistivity: r ≥ 18 MV cm-1) was used to prepare solutions.
All electrochemical experiments were performed on a CHI 830B electrochemical workstation (CH Instruments,China) connected to a personal computer in a traditional three-electrode configuration. A glassy carbon electrode (GCE,d = 3 mm),Ag/AgCl (in saturated KCl solution),and a platinum wire were served as working, reference,and counter electrodes,respectively. The current density in this work was recorded as the ratio of current to the geometric area of GCE (0.0707 cm2).
The X-ray diffraction (XRD) patterns were obtained on an X-ray D/max-2200vpc instrument (Rigaku Corporation,Japan) operating at 40 kV and 20 mA,and using Cu K radiation (k = 0.1541 nm). The morphologies and compositions of these as-prepared samples were studied by Philips XL-30 ESEM equipped with an EDS analyzer. The nitrogen adsorption-desorption isotherm was performed on an ASAP 2020 (Micromeritics,USA). Before the measurements,samples were degassed in vacuum at 150 8C for 6 h. The Brunauer-Emmett-Teller (BET) method was utilized to calculate the specific surface areas using the adsorption data. The pore size distribution was derived from the adsorption branches using the Barrett-Joyner-Halenda (BJH) model. X-ray photoelectron spectroscopy (XPS) was measured using Thermo ESCA LAB spectrometer (USA).
2.2. Catalysts synthesisAn appropriate amount of PVP was dissolved in DMF at room temperatureunder stirring until the solutionbecame clear.And then a stoichiometric amount of Co(NO3)2·6H2O and Fe(NO3)3·9H2Owith amolar ratio of 1: 2were added into the above solution to produce a homogeneous precursor solution of 10 wt.% PVP and 15 wt.% metal salts for electrospinning. The precursor solutions were loaded into a plastic syringe equipped with a flat stainless steel needle of 0.9mm in diameter. A high voltage supply was connected to the spinneret (stainless steel needle) and a nickel mesh collector (which was placed 15 cm away from the orifice). The electric voltage was set as 20 kV. CoFe2O4 NFs were prepared by heating the electrospun Co(NO3)2/Fe(NO3)3/PVP fibers at 800˚C for 2 h in air. For comparison, NiFe2O4 and Fe2O3 NPs,Co3O4 NFs,and NiO NFs were also prepared using the same procedures.
2.3. Preparation of modified electrodesPrior tomodification,GCE was polished carefully with 1.00,0.30, and 0.05 mm alumina powders and then cleaned with HNO3 (1:1), ethanol,and deionized water sequentially. The catalyst ink was prepared by mixing 3 mg of the catalyst powders into a 1 mL Nafion solution (0.5 wt%)with 45 min of ultrasonication. Then 10 mL of the catalyst ink was dropped onto the cleanly washed GCE and dried under an infrared lamp before electrochemical experiments.
3. Results and discussion 3.1. CharacterizationThe obtained Fe2O3 NPs (Fig. 1A) show the characteristic spheroidicity and the average diameter size is larger than 300 nm. In contrast,both Co3O4 NFs and NiO NFs show the diameters less than 200 nm (as shown in Fig. S1 in Supporting information). Moreover,these Co3O4 NFs and NiO NFs all display the classical fiber structures. Meanwhile,both the CoFe2O4 NFs (Fig. 1B) and NiFe2O4 NFs (Fig. 1C) also display the special fiber morphology and hierarchical networks and possess abundant porous structures in the films. Thus,both Co and Ni appear to be conducive to the formation of fiber structures of MFe2O4 samples. Furthermore,the diameters of the CoFe2O4 and NiFe2O4 NFs range from 100 to 200 nm,and they all show the cross-linked reticular structures. On the other hand,a TEM image displays that the CoFe2O4 NFs are composed of irregular grains (as shown in Fig. S2[21TD$DIF] in Supporting information). The average diameter size of Fe2O3 NFs is larger than 300 nm. The intervals among the NFs result in a large accessible surface area and an increased number of exposed catalytic active sites,which is favorable for the full utilization of the MFe2O4 NFs. Energy dispersive X-ray spectroscopy (EDS) was also conducted for all three samples. It is clear that C,O,Fe,andMwere all detected in the EDS spectra of MFe2O4 NFs (Fig. 1D-F) and their content was also accurately recorded in the corresponding images. It is clear that the molar ratios of Co:Fe and Ni:Fe are close to 1:2 for both CoFe2O4 and NiFe2O4 NFs,in good agreement with the reagent ratios and the stoichiometric ratio of M:Fe in MFe2O4. However,the ratios of O element in both CoFe2O4 and NiFe2O4 NFs are 4 times larger than those of Co and Ni elements. The redundant O element is from the surviving carbons.

|
Download:
|
| Fig. 1.SEM images of the Fe2O3 NFs (A), CoFe2O4 NFs (B), and NiFe2O4 NFs (C). EDX spectra for the Fe2O3 NFs (D), CoFe2O4 NFs (E), and NiFe2O4 NFs (F). | |
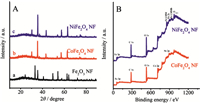
The typical X-ray diffraction (XRD) patterns of the as-prepared Fe2O3 NPs,CoFe2O4 NFs,and NiFe2O4 NFs all present typical features of spinel-type Fe2O3,CoFe2O4,and NiFe2O4 crystals,as shown in Fig. 2A. For the Fe2O3 NFs (curve a),all the reflection peaks appear in the representative XRD patterns can be well indexed to the pure corundum structure of the Fe2O3 phase (JCPDS: 33-0664) [15]. Diffraction peaks at 2θ values of 30.08,35.68,43.48,57.28,and 62.88 are observed for CoFe2O4 NFs (curve b),which correspond to the lattice planes of (2 2 0),(3 1 1),(4 0 0),(5 1 1),and (4 4 0) of CoFe2O4 phase, respectively,indicatingthe formationofCoFe2O4 crystallites (JCPDS: 22-1086) [16]. Similarly,the reflection peaks appear in the representative XRD patterns of NiFe2O4 NFs (curve c) can be well indexed to the pure corundum structure of the NiFe2O4 phase (JCPDS: 10-0325) [2]. X-ray photo-electron spectroscopy (XPS) measurements were also conducted to evaluate the surface chemical bonding states and compositions of these samples,those XPS survey spectra are shown in Fig. 2B. The peaks located at the binding energies of about 530,284,and 710-725 eV in all samples are ascribed to the O 1s,C 1s,and Fe 2p,respectively.
On the other hand,these MFe2O4 NFs-based materials also show their characteristic peaks located between 755 and 800 eV (Co 2p) for CoFe2O4 NFs and 850-875 eV (Ni 2p) for NiFe2O4 NFs. The high resolution XPS spectra of Fe 2p andM2p for both CoFe2O4 NFs and NiFe2O4 NFs are also shown in Fig. 3. As for the Fe 2p core level peaks (Fig. 3A),one can see from that the iron exists mainly in the Fe(III) oxidation state,with only residual Fe(II) oxidation state for the two samples. Meanwhile,as shown in Fig. 3B,the Co 2p peaks of CoFe2O4 NFs consist of two spin-orbit doublets characteristics of Co 2p3/2 (780.0 eV,Co2+ in Oct-site; 781.7 eV, Co2+ in Tet-site; 783.3 eV,Co3+ in Oct-site) and Co 2p1/2 (795.2 eV, Co2+ in Oct-site and 796.2 eV,Co2+ in Tet-site) and two shakeup satellites [2]. It is anticipated that the transition of solid state redox couples of Co3+/Co2+ and Fe3+/Fe2+ in spinel CoFe2O4 structure may be responsible for the electro-catalytic performance of the CoFe2O4 NFs. Similar to the Co 2p peaks in the CoFe2O4 NFs,the Ni 2p XPS spectra recorded from the NiFe2O4 NFs sample indicate that Ni3+ and Ni2+ exist in the NiFe2O4 NFs according to the literature reports [17].
|
Download:
|
| Fig. 2.(A) Wide-angle XRD patterns of the Fe2O3 (curve a), CoFe2O4 (curve b), and NiFe2O4 (curve c) NFs. (B) XPS survey spectra of the CoFe2O4 and NiFe2O4 NFs. | |

|
Download:
|
| Fig. 3.High resolution XPS spectra of Fe 2p (A) for the CoFe2O4 and NiFe2O4 NFs. High resolution XPS spectra of Co 2p (B) for the CoFe2O4 NFs and Ni 2p (C) for the NiFe2O4 NFs. | |
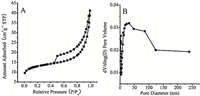
N2 adsorption-desorption measurements were performed to further investigate the porous nature of the CoFe2O4 NFs (as shown in Fig. 4A). The N2 adsorption isotherm shows type III isotherm having a hysteresis loop at the pressure range of 0.4-1.0 P/P0. Detection results indicate that the CoFe2O4 NFs possess a large BET specific surface area of 61.48 m2 g-1 by virtue of the unique structural features caused by the 3D net-like structure of CoFe2O4 NFs. The pore size distribution was calculated using the BJH method from the desorption branch of the isotherm of the CoFe2O4 NFs (Fig. 4B). One can see that the CoFe2O4 NFs have a wide pore size distribution from 3 to 250 nm,demonstrating the excellent porous structures (including micropores,mesopores,and macropores) of the 3D net-like spinel-type CoFe2O4 NF films. This 3D net-like textural structure can result in more accessible transport channels to effectively transfer electroactive substances/ electrons and increase the density and reactivity of exposed catalytic active sites.
|
Download:
|
| Fig. 4.Nitrogen adsorption–desorption isotherms (A) and pore size distribution curves (B) of the CoFe2O4 NFs. | |
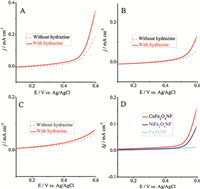
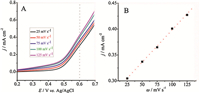
We then explore the oxidation of hydrazine using our catalysts and their detection efficiency. Before the electrochemical experiments, all modified GCEs were scanned in a N2 saturated 0.1 mol/L NaOH solution from -0.2 to +0.7 V vs. Ag/AgCl at a scan rate of 50 mV s-1 for 40 cycles. A comparative study on the electrocatalytic performance of different modified GCEs to hydrazine oxidation was conducted in a 0.1 mol/L NaOH aqueous solution in the absence (dot line) and presence (solid line) of 5.0 mmol L-1 hydrazine by the CV measurement (Fig. 5). After the addition of 5.0 mmol L-1 hydrazine into the 0.1 mol/L NaOH solution,it can be seen that the onset potential (Eonset) of hydrazine oxidation is approximately +0.15 V vs. Ag/AgCl for the CoFe2O4 NFs/Nafion/GCE (Fig. 5A). This value is much more negative than those for the NiFe2O4 NFs/Nafion/GCE (+0.2 V vs. Ag/AgCl; shown in Fig. 5B) and Fe2O3 NPs/Nafion/GCE (+0.25 V vs. Ag/AgCl; shown in Fig. 5C). Those corresponding current density responses (Dj/mA cm-2) of different modified electrodes are calculated and recorded at different potential from the LSV data (Fig. 5D). Furthermore,the corresponding catalytic current responses of Co3O4 and NiO NFsmodified GCEs have also been recorded. The excellent catalytic efficiency of CoFe2O4 and NiFe2O4 NFs proved the superiority of MFe2O4 (as shown in Fig. S3 in Supporting information). Above all,the best hydrazine oxidation efficiency of the resultant CoFe2O4 NFs can be explained by the excellent synergistic effect of Co3+/Co2+ and Fe3+/Fe2+ in the spinel structure,welldeveloped in- and out-of-plane micro/meso/macropores,and accessible transport channels of the CoFe2O4 NFs. LSV measurement was also investigated at different scan rates between 0.2 and 0.7 V vs. Ag/AgCl in a 0.1 mol/L NaOH with 5 mmol L-1 hydrazine for the CoFe2O4 NFs/GCE (Fig. 6A). The dependence of current density at 0.6 V vs. Ag/AgCl to different scan rates was presented in Fig. 6B. One can see that the oxide current density recorded at 0.6 V vs. Ag/AgCl increased linearly with the scan rate in the range from 25 to 125 mV s-1,indicating a surfacecontrolled electrochemical process of hydrazine oxidation on the CoFe2O4 NFs.
|
Download:
|
| Fig. 5.LSV curves of the CoFe2O4 NFs/GCE (A), NiFe2O4 NFs/GCE (B), and Fe2O3 NFs/GCE (C) in absence (dot line) and presence (solid line) of 5.0 mmol L-1 hydrazine in the 0.1 mol/L NaOH solution at a scan rate of 50 mV s-1. (D) The corresponding catalytic current responses of different modified GCEs at different potentials. | |
|
Download:
|
| Fig. 6.(A) LSV measurements for the CoFe2O3 NFs/GCE recorded in 0.1 mol/L NaOH at different scan rates from 25 to 125 mV s-1. (B) The linear relationship of oxidation current densities (recorded at 0.6 V vs. Ag/AgCl) vs. scan rates. | |
For the electrochemical sensing application,the sensing performance of the catalysts is usually evaluated by measuring the current response at fixed potentials versus time after the addition of analytes. Before the test,from the LSV curve without hydrazine,we can see that oxygen evolution reaction (OER) has not appeared at +0.6 V vs. Ag/AgCl for the three samples. However, once the chosen working potential is too positive,the OER process will hold the dominant position [2],which may affect the detection accuracy. Thus,+0.6 V vs. Ag/AgCl was adopted for amperometric i-t tests here. One can see that the amperometric response curve is the typical stair-like i-t curve on the stepwise addition of hydrazine (Fig. 7A). By analyzing the magnified amperometric curve of CoFe2O4 NFs/GCEs,we found that the current response will arrive 90% of the next current density only in 3 s. The calibration curves of CoFe2O4 NFs/GCEs were plotted and there are two linear ranges. In the high hydrazine concentration range (Fig. 7B),a sensitivity of 503 μA cm-2 (mmol L-1)-1 hydrazine (R = 0.999) and a linear range of 0.1-11 mmol L-1are obtained for the CoFe2O4 NFs/GCEs. However,when in a low hydrazine concentration range (Fig. 7C),a detection limit of 1 mmol/L (S/N = 3) can be observed,and the sensitivity increases to 1327 mA cm-2 (mmol L-1)-1 toward hydrazine accompanying a linear range of 0.01-0.1 mmol L-1 . These detection parameters can completely meet the needs of hydrazine detection in different systems and environments. We have also compared the detection parameters of CoFe2O4 NFs with other reported hydrazine sensors in Table 1 and CoFe2O4 NFs compared favorably to other hydrazine sensors. The selectivity of CoFe2O4 NFs was also investigated against the general co-existence of interfering species such as: AA, UA,and DA. As shown in Fig. S[24TD$DIF]4 in Supporting information, the CoFe2O4 NFs produce negligible current signal ratios with the addition of 0.5 mmol L-1 interfering reagents,which indicates the good anti-interference ability of the CoFe2O4 NFs toward electroactive species.

|
Download:
|
| Fig. 7.(A) Amperometric i–t test with the successive addition of hydrazine for the CoFe2O4 NFs/GCE at +0.6 V vs. Ag/AgCl. The corresponding calibration plots at the high concentration levels (B) and low concentration levels (C); (D) stability of the CoFe2O4 NFs/GCE to hydrazine detection every five days in one month. | |
| Table 1 Comparison of the performances of our catalysts toward other reported hydrazine biosensors. |
Finally,we have determined the reproducibility of the CoFe2O4 NFs/GCEs via comparing the detection current density. The amperometric responses of 10 different CoFe2O4 NFs/GCEs to 5 mmol L-1 hydrazine were independently recorded in the same manner and a relative standard deviation (R.S.D.) of 4.25% was obtained. The long-term stability of the CoFe2O4 NFs/GCEs was also explored by measuring the hydrazine solution intermittently. It is clear that the CoFe2O4 NFs/GCE maintains at least 94% of the initial value after one month (Fig. 7D),suggesting its excellent long-term stability.
4. ConclusionWe have synthesized the heterojunction Fe2O3,NiFe2O4,and CoFe2O4-based NFs by electrospinning and the subsequent thermal treatment processes. Characterization results indeed demonstrate the three-dimensional net-like textural structures of these as-electrospun spinel-type MFe2O4 NFs. The film configurations possess abundant micro/meso/macropores on both the surface and within the films. These structures will afford more accessible transport channels to effectively decrease the mass transport resistance and increase the density of exposed catalytic active sites. All these advantages are responsible for the enhanced electro-catalytic performance of these MFe2O4 NFs. When used for hydrazine catalysis and detection,CoFe2O4 NFs show the best catalytic efficiency. The superior catalytic efficiency,excellent stability,low cost,and ease of fabrication render CoFe2O4 NFs very promising materials in developing an electrochemical device for the direct detection of hydrazine.
AcknowledgmentThe authors gratefully acknowledge the Fundamental Research Funds for the Central Universities (No. 12SSXT145).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.10.026.
| [1] | M. Li, L. Liu, Y. Xiong, et al., Bimetallic MCo (M = Cu, Fe, Ni, and Mn) nanoparticles doped-carbon nanofibers synthetized by electrospinning for nonenzymatic glucose detection, Sens. Actuators, B: Chem. 207 (2015) 614-622. |
| [2] | M. Li, Y. Xiong, X. Liu, et al., Facile synthesis of electrospun MFe2O4 (M = Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction, Nanoscale 7 (2015) 8920-8930. |
| [3] | M. Li, C. Han, Y. Zhang, X. Bo, L. Guo, Facile synthesis of ultrafine Co3O4 nanocrystals embedded carbon matrices with specific skeletal structures as efficient non-enzymatic glucose sensors, Anal. Chim. Acta 861 (2015) 25-35. |
| [4] | C.C. Kuo, W.J. Lan, C.H. Chen, Redox preparation of mixed-valence cobalt manganese oxide nanostructured materials: highly efficient noble metal-free electrocatalysts for sensing hydrogen peroxide, Nanoscale 6 (2014) 334-341. |
| [5] | H. Zhu, S. Zhang, Y.X. Huang, L. Wu, S. Sun, Monodisperse MxFe3-xO4 (M = Fe, Cu, Co, Mn) nanoparticles and their electrocatalysis for oxygen reduction reaction, Nano Lett. 13 (2013) 2947-2951. |
| [6] | D. Yu, J. Yao, L. Qiu, et al., In situ growth of Co3O4 nanoparticles on alpha-MnO2 nanotubes: a new hybrid for high-performance supercapacitors, J. Mater. Chem. A 2 (2014) 8465-8471. |
| [7] | A. Afkhami, H. Khoshsafar, H. Bagheri, T. Madrakian, Preparation of NiFe2O4/ graphene nanocomposite and its application as a modifier for the fabrication of an electrochemical sensor for the simultaneous determination of tramadol and acetaminophen, Anal. Chim. Acta 831 (2014) 50-59. |
| [8] | L. Luo, Y. Zhang, F. Li, et al., Enzyme mimics of spinel-type CoxNi1-xFe2O4 magnetic nanomaterial for eletroctrocatalytic oxidation of hydrogen peroxide, Anal. Chim. Acta 788 (2013) 46-51. |
| [9] | L. Li, Y.Q. Zhang, X.Y. Liu, et al., One-dimension MnCo2O4 nanowire arrays for electrochemical energy storage, Electrochim. Acta 116 (2014) 467-474. |
| [10] | C.L. Zhang, S.H. Yu, Nanoparticles meet electrospinning: recent advances and future prospects, Chem. Soc. Rev. 43 (2014) 4423-4448. |
| [11] | J. Huang, D. Wang, H. Hou, T. You, Electrospun palladium nanoparticle-loaded carbon nanofibers and their electrocatalytic activities towards hydrogen peroxide and NADH, Adv. Funct. Mater. 18 (2008) 441-448. |
| [12] | G. Wang, Q. Dong, Z. Ling, et al., Hierarchical activated carbon nanofiber webs with tuned structure fabricated by electrospinning for capacitive deionization, J. Mater. Chem. 22 (2012) 21819-21823. |
| [13] | R. Pauliukaite, S.B. Hocevar, E.A. Hutton, B. Ogorevc, Novel electrochemical microsensor for hydrogen peroxide based on iron-ruthenium hexacyanoferrate modified carbon fiber electrode, Electroanalysis 20 (2008) 47-53. |
| [14] | M.U.A. Prathap, V. Anuraj, B. Satpati, R. Srivastava, Facile preparation of Ni(OH)2- MnO2 hybrid material and its application in the electrocatalytic oxidation of hydrazine, J. Hazard. Mater. 262 (2013) 766-774. |
| [15] | X. Cao, N. Wang, A novel non-enzymatic glucose sensor modified with Fe2O3 nanowire arrays, Analyst 136 (2011) 4241-4246. |
| [16] | Z. Zhang, Y. Wang, M. Zhang, et al., Mesoporous CoFe2O4 nanospheres crosslinked by carbon nanotubes as high-performance anodes for lithium-ion batteries, J. Mater. Chem. A 1 (2013) 7444-7450. |
| [17] | M. Li, X. Bo, Y. Zhang, et al., Cobalt and nitrogen co-embedded onion-like mesoporous carbon vesicles as efficient catalysts for oxygen reduction reaction, J. Mater. Chem. A 2 (2014) 11672-11682. |
| [18] | B. Fang, Y. Feng, M. Liu, et al., Electrocatalytic oxidation of hydrazine at a glassy carbon electrode modified with nickel ferrite and multi-walled carbon nanotubes, Microchim. Acta 175 (2011) 145-150. |
| [19] | M. Kamyabi, O. Narimani, H.H. Monfared, Electrocatalytic oxidation of hydrazine using glassy carbon electrode modified with carbon nanotube and terpyridine manganese(II) complex, J. Electroanal. Chem. 644 (2010) 67-73. |
| [20] | J. Li, X. Lin, Electrocatalytic oxidation of hydrazine and hydroxylamine at gold nanoparticle—polypyrrole nanowire modified glassy carbon electrode, Sens. Actuators, B: Chem. 126 (2007) 527-535. |
| [21] | Y. Ni, J. Zhu, L. Zhang, J. Hong, Hierarchical ZnO micro/nanoarchitectures: hydrothermal preparation, characterization and application in the detection of hydrazine, CrystEngComm 12 (2010) 2213-2218. |
| [22] | G. Wang, A. Gu, W. Wang, et al., Copper oxide nanoarray based on the substrate of Cu applied for the chemical sensor of hydrazine detection, Electrochem. Commun. 11 (2009) 631-634. |
| [23] | J. Zheng, Q. Sheng, L. Li, Y. Shen, Bismuth hexacyanoferrate-modified carbon ceramic electrodes prepared by electrochemical deposition and its electrocatalytic activity towards oxidation of hydrazine, J. Electroanal. Chem. 611 (2007) 155-161. |
| [24] | J. Zhang, H. Liu, M. Dou, et al., Synthesis and characterization of Co3O4/multiwalled carbon nanotubes nanocomposite for amperometric sensing of hydrazine, Electroanalysis 27 (2015) 1188-1194. |
| [25] | K.K. Lee, P.Y. Loh, C.H. Sow, W.S. Chin, CoOOH nanosheet electrodes: simple fabrication for sensitive electrochemical sensing of hydrogen peroxide and hydrazine, Biosens. Bioelectron. 39 (2013) 255-260. |
| [26] | L. Zheng, J.F. Song, Ni(II)-baicalein complex modified multi-wall carbon nanotube paste electrode toward electrocatalytic oxidation of hydrazine, Talanta 79 (2009) 319-326. |
| [27] | G. Chang, Y. Luo, W. Lu, et al., Immobilization of Au nanoparticles on Au electrode for hydrazine detection: using thiolated single-stranded DNA as a linker, Thin Solid Films 519 (2011) 6130-6134. |
| [28] | S. Shukla, S. Chaudhary, A. Umar, G.R. Chaudhary, S. Mehta, Tungsten oxide (WO3) nanoparticles as scaffold for the fabrication of hydrazine chemical sensor, Sens. Actuators, B: Chem. 196 (2014) 231-237. |
| [29] | B.Šljukić, C.E. Banks, A. Crossley, R.G. Compton, Iron(III) oxide graphite composite electrodes: application to the electroanalytical detection of hydrazine and hydrogen peroxide, Electroanalysis 18 (2006) 1757-1762. |
 2015, Vol.26
2015, Vol.26 



