Reactive oxygen species (ROS) are a class of radical or nonradical oxygen-containing molecules that show high reactivity to biomolecules [1]. Although generation of ROS is often simply regarded as representing oxidative stress,each ROS has unique chemical characteristics in terms of chemical reactivity and lifetime in aqueous solution and,therefore,may play a distinct role in biological systems [2]. Hydrogen peroxide (H2O2) exhibits relatively mild reactivity among ROS and has attracted intense interest in recent years. It appears to be involved in signal transduction by reversible oxidation of proteins,such as phosphatases and thioredoxins,in a tightly regulated manner. The fast and accurate detection of H2O2 has profound applications in pharmaceutical,clinical,food industry,environmental analysis and other fields [3, 4]. Thus,numerous analytical methods have been applied for the detection of H2O2 such as fluorescence [5, 6], chemiluminescence [7, 8],and electrochemical methods [9, 10, 11].
Among these methods,the electrochemical technique is the most studied because of its simplicity,fast response for analysis, low detection limit,and low costs [12]. Sombers and co-workers have detected rapid H2O2 fluctuations at an uncoated carbon fiber microelectrode by fast scan cyclic voltammetry in vitro and in brain slices. Recently,several boronate-based fluorescence probes capable of detecting H2O2 have been reported,such as peroxyfluor- 1,peroxyresorufin-1 and peroxyxanthone-1 [13, 14, 15, 16, 17]. These fluorescent probes are cell membrane-permeable,and able to monitor the intracellular H2O2 concentration changes in living cells. At the same time,chemiluminescence (CL)-based assays possess several advantages such as simplicity in instrumentation [18],high sensitivity,and wide linear range. Since no external light source is used for excitation in chemiluminescent approaches,nonspecific signals caused by external light excitation as often observed in fluorescence-based measurements can be minimized. Suzuki et al. has reported a luciferin-based long-wavelength chemiluminescent based probe,KEIO-BODIPY-imidazopyrazine,which exhibited the strong response toward H2O2.
Although various techniques in H2O2 sensing have been installed to enhance sensitivity,selectivity,and the dynamic working range,these approaches mainly follow the paradigm that is still dominating traditional probe design: one probe for one detection method. Therefore,the application of the probe will be significantly limited by the requirement of instrument and complicated samples containing the different interfering species. As an alternative strategy,here we reported a mutli-signaling probe,Ru[(bpy)2luminol-bpy](PF6)2,that can detect H2O2 in three sensing channels including photoluminescence,chemiluminiscence and eletrochemiluminiscence.
2. Experimental 2.1. Materials and instrumentation5-Amino-2,3-dihydrophthalazine-1,4-dione,hydrogen peroxide, horseradish peroxidase (HRP) was purchased from Aladdin. 4-Methyl-4'-methyl-2,2'-bipyridine was purchased from Sigma- Aldrich. [Ru(bpy)2(COOH-bpy)](PF6)2 was synthesized by using the literature methods [19]. Unless otherwise stated,all chemical materials were purchased from commercial sources and used without further purification.
1 H NMR and 13C NMR spectra were measured on a Bruker Avance spectrometer (400 MHz for 1H NMR and 100 MHz for 13C NMR). Mass spectra were recorded on a HP1100 LC/MSD MS spectrometer. Absorption spectra were measured on a Perkin- Elmer Lambda 35 UV-vis spectrometer. Elemental analysis was carried out on a Vario-EL analyser. Photoluminescence spectra were measured on a Perkin-Elmer LS-50 luminescence spectrometer. All the ECL measurements were carried out on an ECL insECL cell at room temperature. All measurements of chemiluminiscence were carried out IFFM-D flow injection chemiluminescence analyzer and IFFS-A instrument system (Remex Electronics Instrument Co.,Ltd.).
2.2. Synthesis of [Ru(bpy)2(luminol-bpy)](PF6)2A solution of [Ru(bpy)2(COOH-bpy)](PF6)2 (93.6 mg, 0.1 mmol) in 5 mL SOCl2 was refluxed for 5 h under an argon atmosphere. After removing the excess SOCl2 by distillation under reduced pressure,the residue was dried in vacuum for 2 h,and dissolved in 30 mL absolute CH3CN. The solution was added to a mixture of luminal (17.5 mg,0.1 mmol) and Et3N (21 L,0.15mmol). The mixture was refluxed for 12 h. The solvent was evaporated,and the residue was purified by silica gel column chromatography using CH3CN-H2O-KNO3 (sat.) (100:20:1,v/v/v) as eluent. The fractions containing the target product were collected,and the solvent was evaporated. The resulting solid was dissolved in a small amount of CH3CN- H2O(1:1),and a saturated solution of NH4PF6 was added to give a red precipitate. The product was filtered and washed with small amount of water. Compound [Ru(bpy)2(luminolbpy)]( PF6)2 was obtained as a red powder (75.89 mg,70.4% yield). 1H NMR (400MHz,CD3CN): δ 2.54 (s,3H),7.27(m,1H), 7.38-7.43(m,4H),7.57(m,1H),7.70(s,1H),7.74(m,3H),7.78(s, 1H),7.81(m,2H),7.97(m,1H),8.04-8.09(m,4H),8.51(d,5H, J(H,H) = 4 Hz),8.97(s,1H),8.99(m,1H). 13C NMR (100 MHz, CD3CN): δ 19.97,117.04,118.96,121.24,123.44,124.00,124.03, 124.08,125.32,127.29,127.34,127.37,127.39,128.59,134.81, 137.70,150.54,150.59,151.28,151.47,151.49,152.63,155.63, 156.51,156.62,156.73,158.25,161.52. ESI-MS (m/z): 932.0 ([M-PF6]+),393.4 ([M-2PF6]2+). Elemental analysis (%) calcd. for C40H31F12N9O3P2Ru·2H2O: C 43.17,H 3.17,N 11.33; found (%): C 43.12,H 3.03,N,11.13.
2.3. Chemiluminiscence measurementsA mixture of [Ru(bpy)2(luminol-bpy)](PF6)2 solution (1.0 μmol/L),HRP (1.0 μmol/L) and different concentrations of H2O2 (0.1 μmol/L,1.0 μmol/L,2.0 μmol/L,4.0 μmol/L,6.0 μmol/L, 8.0 μmol/L,10 μmol/L,12 μmol/L,28 μmol/L,40 μmol/L, 50 μmol/L,60 μ mol/L,70 μ mol/L) was injected into injection port,respectively. The rotate speed of main and vice-peristaltic pump were set as 20 and 15 r/min,respectively,and CL intensities of the solutions were determined on chemiluminescence analyzer.
2.4. ECL measurements[Ru(bpy)2(luminol-bpy)](PF6)2 (10 μmol/L) and HRP (1.0 μmol/L) was stirred with different concentrations of H2O2 (0 μmol/L,5 μmol/L,15 μmol/L,20 μmol/L,25 μmol/L) at room temperature for 30 min in the PBS buffer (25 mmol/L,pH 12.0), respectively. The glassy carbon (3.0 mm in diameter) electrode and KCl saturated Ag/AgCl electrode were used working electrode and reference electrode,respectively,and a platinum wire (0.3 mm in diameter) was used as the auxiliary electrode. Before measurements,the glassy carbon working electrode was soaked in 10% HNO3 in an ultrasonic water bath for 1 min,then polished by an Al2O3 slurry,and thoroughly rinsed with deionized water for 1 min. The voltage of the photomultiplier tube was set at 900 V in the detection process while collecting the ECL signals.
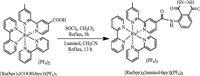
3. Results and discussion 3.1. Design and synthesis of the mutli-signaling probeA novel multi-signaling probe for H2O2,Ru[(bpy)2luminolbpy]( PF6)2,was developed by conjugating luminal with a luminescent Ru(II) complex. The luminescent Ru(II)-polypyridyl complexes has attracted much attention due to their abundant photophysical,photochemical,and electrochemical properties, such as visible-light excitation and emission with large Stokes shifts,high photo- and chemical stabilities,low cytotoxicity,good water-solubility,and high PL and ECL response efficiency [20- 23]. In addition,the output signals of Ru(II)-polypyridyl complexes can be modulated by appropriate modification of the pyridine moiety. At the same time,it is well known that luminal is a specifically reactive group for H2O2,and has been widely used for the development of chemiluminescent probe for H2O2 detection. Ru[(bpy)2luminol-bpy](PF6)2 was successfully synthesized as shown in Scheme 1,and the structure of the probe was well confirmed by NMR spectroscopy,MS,and elementary analyses.
|
Download:
|
| Scheme. 1. Synthesis of Ru[(bpy)2luminol-bpy](PF6)2. | |
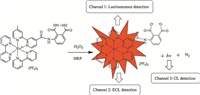
After conjugated with a luminal moiety,the metal-to-ligand charge transfer (MLCT) emission of the Ru(II) complex is effectively corrupted by the electron of N atoms via an intramolecular photoinduced electron transfer (PET) process. Ru[(bpy)2luminolbpy]( PF6)2 exhibited weak luminescence at 645 nm. It is well known that the luminal moiety can react with ROS,which are generated by the reaction of H2O2 with HRP,to generate a high energy species that decomposes to give an excited molecule with loss of nitrogen molecule [24]. Therefore,after reacting with H2O2 in the presence of HRP to trigger the cleavage of the N atoms,the PET process is eliminated,so that the luminescence of the Ru(II) complex can be turned on (Fig. 1).
|
Download:
|
| Fig. 1.Reaction of Ru[(bpy)2luminol-bpy](PF6)2 with H2O2 in the presence of HRP. | |
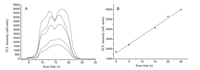
To investigate the luminescence response of the Ru(II) complex to H2O2,the emission spectra of [Ru[(bpy)2(luminal-bpy)](PF6)2 (10 μmol/L) in the presence of HRP (1.0 μmol/L) and different concentrations of H2O2 were recorded in 25 mmol/L PBS buffer of pH 12.0. As shown in Fig. 2A,the complex [Ru(bpy)2(luminalbpy)]( PF6)2 exhibited weak luminescence at 645 nm. Upon reaction with different concentrations of H2O2,the luminescence intensity of the complex was increased gradually. In addition,the dose-dependent luminescence enhancement showed a good linearity in the H2O2 concentration range of 0-35 μmol/L (Fig. 2B),suggesting [Ru[(bpy)2(luminal-bpy)](PF6)2 can quantitatively detect H2O2 by using luminescence methods.

|
Download:
|
| Fig. 2.A. Luminescence emission of [Ru(bpy)2luminal-bpy)](PF6)2 (10 μmol/L) upon addition of H2O2 (0–35 μmol/L) and HRP (1 μmol/L) for 5 min at 25 °C in 25 mmol/L PBS buffer (pH 12.0). λex = 450 nm. B. The calibration curve for luminescence detection of H2O2. | |
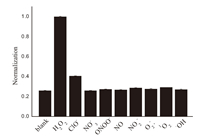
Furthermore,[Ru[(bpy)2(luminal-bpy)](PF6)2 exhibited the good selectivity for H2O2. As shown in Fig. 3,the probe did not give any observable luminescence responses to the addition of other ROS/RNS,such as ClO-,NO3 -,·OH,NO,NO2 -,ONOO-,1O2 and O2 -.,while the luminescence intensity was remarkably increased after [Ru[(bpy)2(luminal-bpy)](PF6)2 was reacted with H2O2. These results demonstrate that the response of the probe to H2O2 is highly specific by using luminescence methods.
|
Download:
|
| Fig. 3.PL intensities of Ru[(bpy)2luminol-bpy](PF6)2 (10 μmol/L) upon reaction with various ROS/RNS (60 μmol/L) in 25 mmol/L PBS buffer with pH 12.0. | |
In the ECL case,the similar phenomenon might also occur since the excited-state electron can be also withdrawn by the electron acceptor group to retain the complex in the ECL-off state,while its ECL behavior should be turned on after the cleavage reaction induced by H2O2 and HRP. The ECL intensity of [Ru[(bpy)2(luminolbpy)(PF6)2 was investigated upon addition of different concentration of H2O2 in 25 mmol/L PBS buffer (pH 12.0) containing 10 mmol/L of TPrA. As shown in Fig. 4A,when the cyclic potential was scanned from 0.2 V to 1.8 V and then backed from 1.8 V to 0.2 V,a typical ECL emission from the excited-state of the Ru(II) complex appeared at ~1.27 V,which was increased followed by the increase of H2O2 concentration. By plotting the ECL intensity versus the H2O2 concentration,a good linear calibration curve with a dynamic range of 0-25 μmol/L was obtained (Fig. 4B). This result indicates that [Ru(bpy)2(luminol-bpy)](PF6)2 can be also used as a ECL probe for the quantitative detection of H2O2.
|
Download:
|
| Fig. 4.A. ECL intensity responses of [Ru(bpy)2(luminal-bpy)](PF6)2 (10 μmol/L) upon addition of H2O2 (0–25 μmol/L) and HRP (1.0 μmol/L) at roomtemperature in 25 μmol/L PBS buffer (pH 12.0) containing 10 mmol/L of TPrA. Photomultiplier tube voltage: 900 V; B. The calibration curve for the ECL detection of H2O2. | |
A unique advantage of chemiluminescence detection technique is that can effectively lower the signal-to-noise ratio and improve the sensitivity due to an excitation source is not needed for chemiluminescence detection,and the signal interferences from the background fluorescence that is triggered by an external excitation source can be avoided. The most widely used chemiluminescence system is a luminol/hydrogen peroxide reaction catalyzed by horseradish peroxidase. Here a Ru(II) complex containing a luminal moiety,Ru[(bpy)2luminolbpy]( PF6)2,was evaluated for the chemiluminescence detection of H2O2. The chemiluminescence spectra of Ru[(bpy)2luminolbpy]( PF6)2 (1.0 μmol/L) was recorded in the presence of H2O2 (0.1- 70 mmol/L) and HRP (1.0 μmol/L) in 25 mmol/L PBS buffer (pH 12.0). As shown in Fig. 5A,upon addition of H2O2,chemiluminescence intensity of Ru[(bpy)2luminol-bpy](PF6)2 was clearly increased. The dose-dependent luminescence enhancement followed a good linear relationship with H2O2 concentrations in a range of 0-7.0 mmol/L,and a detection limit of 0.6 mmol/L was obtained (Fig. 5B). This result indicates that Ru[(bpy)2luminolbpy]( PF6)2 can be also used as a chemiluminescence probe for the quantitative detection of H2O2 at a low micromolar concentration level.

|
Download:
|
| Fig. 5.Chemilunminescence spectra of Ru[(bpy)2luminol-bpy](PF6)2 (1.0 μmol/L) upon addition of H2O2 (0.1–70 μmol/L) and HRP (1.0 μmol/L) in 25 mmol/L PBS buffer (pH[3TD$DIF] 12.0); B. The calibration curve for the chemiluminescence detection of H2O2. | |
In conclusion,a multifunction Ru(II) complex,luminal- bpy)](PF6)2,has been developed as a probe for H2O2 by using triple-channel detection. Based on a luminal mioety/hydrogen peroxide reaction catalyzed by horseradish peroxidase,the novel probe can detect H2O2 in the three sensing channels including photoluminescence,chemiluminiscence and eletrochemiluminiscence. The quantitative assays for H2O2 in aqueous solutions using [Ru[(bpy)2(Luminal-bpy)](PF6)2 were preliminarily established with PL,ECL and CL signal output modes,respectively and its feasibility of practical application was studied on. We believe that the present approach could provide a useful strategy to design and synthesize multifunctional probes for biomolecules.
AcknowledgmentThis project was supported by the National Natural Science Foundation of China (No. 21205009) and the Fundamental Research Funds for the Central Universities (No. DUT15ZD116).
| [1] | B. Autreaux, M.B. Toledano, ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis, Nat. Rev. Mol. Cell Biol. 8 (2007) 813-824. |
| [2] | C.C. Winterbourn, Reconciling the chemistry and biology of reactive oxygen species, Nat. Chem. Biol. 4 (2008) 278-286. |
| [3] | H. Chen, H. Wang, X.J. Qin, et al., A bestatin-based fluorescent probe for aminopeptidase N cell imaging, Chin. Chem. Lett. 26 (2015) 513-516. |
| [4] | F.Y. Zhang, Z.H. Wang, Y.Z. Zhang, et al., Simultaneous electrochemical determination of uric acid, xanthine and hypoxanthine based on poly(L-arginine)/graphene composite film modified electrode, Talanta 93 (2012) 320-325. |
| [5] | F. Wen, Y.H. Dong, L. Feng, et al., Horseradish peroxidase functionalized fluorescent gold nanoclusters for hydrogen peroxide sensing, Anal. Chem. 83 (2011) 1193-1196. |
| [6] | K. Hirakawa, Fluorometry of hydrogen peroxide using oxidative decomposition of folic acid, Anal. Bioanal. Chem. 386 (2006) 244-248. |
| [7] | K. Wang, Q. Liu, X.Y. Wu, et al., Graphene enhanced electrochemiluminescence of CdS nanocrystal for H2O2 sensing, Talanta 82 (2010) 372-376. |
| [8] | A. Tahirović, A.Có pra, E. Omanović-Miklicanin, et al., A chemiluminescence sensor for the determination of hydrogen peroxide, Talanta 72 (2007) 1378-1385. |
| [9] | L.B. Zhang, S.R. Yang, J.Q. Wang, et al., A facile preparation and electrochemical properties of nickel based compound-graphene sheet composites for supercapacitors, Chin. Chem. Lett. 26 (2015) 522-528. |
| [10] | J.J. Zhang, Y.G. Liu, L.P. Jiang, et al., Synthesis, characterizations of silica-coated gold nanorods and its applications in electroanalysis of hemoglobin, Electrochem. Commun. 10 (2008) 355-358. |
| [11] | J.P. Li, Y.P. Li, G. Wei, et al., Highly sensitive molecularly imprinted electrochemical sensor based on the double amplification by an inorganic prussian blue catalytic polymer and the enzymatic effect of glucose oxidase, Anal. Chem. 84 (2012) 1888-1893. |
| [12] | S.Y. Xu, B. Peng, X.Z. Han, A third-generation H2O2 biosensor based on horseradish peroxidase-labeled Au nanoparticles self-assembled to hollow porous polymeric nanopheres, Biosens. Bioelectron. 22 (2007) 1807-1810. |
| [13] | M.C.Y. Chang, A. Pralle, E.Y. Isacoff, C.J. Chang, A selective, cell-permeable optical probe for hydrogen peroxide in living cells, J. Am. Chem. Soc. 126 (2004) 15392-15393. |
| [14] | E.W. Miller, A.E. Albers, A. Pralle, et al., Boronate-based fluorescent probes for imaging cellular hydrogen peroxide, J. Am. Chem. Soc. 127 (2005) 16652-16659. |
| [15] | D. Srikun, E.w. Miller, D.W. Domaille, et al., An ICT-based approach to ratiometric fluorescence imaging of hydrogen peroxide produced in living cells, J. Am. Chem. Soc. 130 (2008) 4596-4597. |
| [16] | B.C. Dickinson, C.J. Chang, A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells, J. Am. Chem. Soc. 130 (2008) 9638-9639. |
| [17] | E.W. Miller, O. Tulyathan, E.Y. Isacoff, et al., Molecular imaging of hydrogen peroxide produced for cell signaling, J. Nat. Chem. Biol. 3 (2007) 263-267. |
| [18] | L.X. Zhao, L. Sun, X.G. Chu, Chemiluminescence immunoassay, TrAC: Trends Anal. Chem. 28 (2009) 404-415. |
| [19] | R. Zhang, B. Song, Z.Q. Ye, et al., Highly sensitive and selective phosphorescent chemosensors for hypochlorous acid based on ruthenium(II) complexes, Biosens. Bioelectron. 50 (2013) 1-7. |
| [20] | W.Z. Zhang, R. Zhang, Z.Q. Ye, et al., Photoluminescent and electrochemiluminescent dual-signaling probe for bio-thiols based on a ruthenium(II) complex, Anal. Chim. Acta 740 (2012) 80-87. |
| [21] | D.M. Josefina, F. Oscar, E. Roberto, A ruthenium-rhodamine complex as an activatable fluorescent probe, Anal. Chem. 82 (2010) 6259-6264. |
| [22] | S.H. Fan, J. Shen, H. Wu, et al., A highly selective turn-on colorimetric and luminescence sensor based on a triphenylamine-appended ruthenium(II) dye for detecting mercury ion, Chin. Chem. Lett. 26 (2015) 580-584. |
| [23] | B.T. Tamaddoni, A.N. Kharat, S. Zamanian, Chiral electron deficient ruthenium helical coordination polymer as a catalyst for the epoxidation of substituted styrenes, Chin. Chem. Lett. 26 (2015) 137-140. |
| [24] | X. Huang, J. Ren, Gold nanoparticles based chemiluminescent resonance energy transfer for immunoassay of alpha fetoprotein canace marker, Anal. Chim. Acta 686 (2011) 115-120. |
 2015, Vol.26
2015, Vol.26 


