Traditional studies of cellular processes are usually carried out with a large number of cells. The difference between individual cells is mostly masked by cellular information that is measured as averages of large populations [1]. Therefore,developing the analysis at the single cell level is of significance for cell researching. A promising approach to the single-cell analysis is microfluidics, which is characterized by manipulation of small volume fluids and integration of multiple functional units [2]. Microfluidics can provide rapid,accurate,and cost-effective methods for single-cell analysis [3],including the analysis of protein [4] and genes [5] in a single cell. In some cases,the cells need to be isolated for investigating their responses to stimuli or extracellular secretion. Obviously,droplet microfluidics,which has the potential to compartmentalize individual cells into nanoliter or smaller droplets for biochemical analysis [4],can not only meet the requirement above,but also characterize the single-cell analysis by minimal sample dilution,higher sensitivity,shorter reaction time, higher throughput and no cross contamination. The single-cell encapsulation methods based on droplet microfluidics mainly include active encapsulation and passive encapsulation. The former is to actively encapsulate cells in droplets with the aid of external forces,such as optical trapping [6],electric field force [7], acoustic field force [8],pneumatic valves [9] and aspirating force via tapered capillary [10]. It is characterized by accurate targeting of individual cells,and therefore a higher efficiency of single-cell encapsulation in droplets is acquired. However,employing external forces increases the complexity of manipulating system without a corresponding increase in throughput. The latter method is also named hydrodynamic method,which utilizes pressuredriven flow in simple microfluidic configurations for generating auqeous droplets and at the same time entrapping single cells into droplets. The number of cells in one droplet mainly depends on the concentration of cell suspension,and the encapsulation process usually follows Poisson statistics [11]. For example,when the multiple-cell droplets was decreased to 4%,the corresponding ratio of the single-cell droplets was down to 22% and the majority of droplets contained no cell at all [11, 12]. The hydrodynamic method is extremely simple; however,the efficiency of single-cell encapsulation is still insufficient. To solve this problem,some researchers tried to add a sorting step after the stochastic encapsulation of cells [12, 13]. They separated the single-cell droplets from the ones with multiple cells or no cells. Although the efficiency of single-cell encapsulation of the improved hydrodynamtic methods increased to some extent,the encapsulation throughput was still kept at a low level,and the device was more complicated for it had to integrate with other functional modules. Other researchers performed special controlling processes before droplet generation,such as regulating the cell density [14],evenly spacing the travelling cells [15] and ordering cells by Dean flows in a curved microchannel [16]. Regulating the cell density before droplet formation still suffered from the low throughput. The single-cell encapsulation by the aid of spacing cells and ordering cells obained good performances both in efficiency and throughput, although special geometries and subtle regulation of flowrates were needed.
Here,we aim at developing a simple hydrodynamic method to encapsulate single cells in droplets with high efficiency and throughput. The production rate of droplets could be increased by parallelizing droplet generators [17, 18]. The passive repeated breakup of droplets was a simple strategy using a splitting array [19]. Based on the array of droplet splitting structures,we explored a single-cell encapsulation method with high throughput. A large droplet with multiple cells could be repeatedly split until to form the small droplets with single cells.
2. Experimental 2.1. Microfluidic chip design and fabricationTwo types of microfluidic chips were fabricated,respectively,in the experiment. Both of them consisted of flow-focusing water/oil phase channels and a multi-step droplet splitting geometry,in which a main channel was sequentially divided into two branch channels 3 times to form 8 parallel branches (Fig. 1). All of the channels are about 30 μm deep. The width of the main aqueous channel is 120 μm,and the width of the branch channels is, respectively 100 μm,80 μm and 60 μm after each dividing. The oil phase channels contract from 120 μm to 80 μm in width at the flow-focusing junction,and each Y-shaped splitting junction was designed with a nozzle contracting from the width of the upstream channel to the width of the downstream channel. The whole microchannel network has only one aqueous phase (cell suspension) inlet,and one oil phase inlet and 8 outlets. In one type of the microfluidic chips,winding channels were added into the upstream of each junction (Fig. 1b). The microfluidic chip was made of polydimethylsiloxane (PDMS,RTV615,Dow Corning,USA) using the soft lithography technique [20]. Master molds of photoresist (AZ P4620,Clariant,Japan) were fabricated in cleanroom facilities. PDMS layers were replicated from the master molds and bonded with a plain PDMS substrate after air plasma treatment (PDC-32G,Harrick Scientific Co.,USA). The bonding was strengthened by placing the chip on a heating plate at 120 °C for 1 h.

|
Download:
|
| Fig. 1.Schematic diagram of the microfluidic droplet splitting chips. (a) Chip without winding channels; (b) Chip with winding channels. | |
We used yellow food dye solution as the aqueous phase and mineral oil (Sigma,USA) containing 2 wt% Span 80 as the continuous oil phase. Syringe pumps (Model MD-1001,Bioanalytical Systems,USA) were used to introduce the fluids into the microfluidic chip. When the two phases met at the flow-focusing junction,mother aqueous phase droplets were formed. And then the mother aqueous phase droplets were broken into two branch channels at the first Y-shaped splitting junction. After three times of splitting,the mother droplets were finally divided into 8 lines of daughter droplets. The images and movies of droplet generation under different conditions were taken by a high-speed camera (AOS Technologies,Switzerland) mounted on a stereo microscope (Stemi 2000-C,Carl Zeiss,German). The length of the droplets along their central axes was measured by using software of Nis- Elements 3.2.
2.3. Single-cell encapsulationHuman whole blood (supplied and endorsed by volunteers working in a lab of China Medical University) used as the model cell type was diluted by cell suspension medium (pH 7.4) containing 0.85% (w/v) NaCl and 30 mmol/L Tricine-NaOH. And the concentration of cells in human whole blood was regulated using cell separating medium (OptiPrepTM,Axis-shield,Norway). All the reagents were of analytical reagent grade unless specified,and all the solutions were prepared with deionized water and filtered by 0.22 mm filter membrane before used. Two types of microfluidic chips (Fig. 1a and b) were used for the single cell encapsulation experiments. Droplet arrays of food dye solution were formed in continuous oil phase at a flowrate of 1 μL/min for both aqueous phase and oil phase. When the system reached a steady state,the food dye solution was quickly replaced with cell suspension,and the flowrates of two phases were regulated carefully to form single-cell droplets. The process of cell encapsulation was recorded by the high-speed camera mounted on the stereo microscope mentioned above.
3. Results and discussion 3.1. Considerations in the design of the chipThe serial droplet splitting device had been demonstrated to generate monodisperse droplets at a high production rate [17, 19], and it will be extremely attractive if the technique is used for performing the single cell encapsulation. As the technique employs totally passive hydrodynamic driving,the droplet splitting mainly depends on the effect of viscous stresses and interfacial tension, which can be described by the capillary number Ca,Ca = μh/γ, where μ is viscous stresses of the continuous phase,η represents the speed of droplet,and &gama; (N/m) is the interfacial tension between the two immiscible phases. The split ratio is proportional to Ca: the droplets keep spherical state at small Ca value,and tend to break as the Ca value increases. The major factors affecting the split ratio can be summarized as channel dimensions,flow conditions and the interfacial tension of the fluids. The candidate droplets tended to split when their length was larger than the channel width [17, 21],so the width of aqueous channels in different splitting step were designed to stepwise decrease from120 μm to 60 μm to keep daughter droplets large enough to block the channel at every step in this work. Considering the sensitivity of the droplet splitting to the hydrodynamic resistances from the branch channels downstream, the parallel branch channels at the same step were designed of the same dimension to keep the resistances equal. Besides,the disperse phase (cell suspension) used here was different from the homogeneous aqueous disperse phase. The cell suspension was heterogeneous,and the dispersity of cells would seriously impact the number of cells encapsulated in single droplets. In order to deal with the unmanageability and sensitivity of the cell dispersity,many researches had focused on the means to regulate cell movement,such as active mixing by stir [11] and passive mixing by using a curving channel [22]. Here,to regulate the cell disperse state in the passive droplet splitting system, passive mixing structures were arranged before every splitting junction,as shown in Fig. 1b. The mixing structures were designed as winding channels to keep the uniform distribution of cells in the droplets,which had been demonstrated to achieve rapid mixing of the droplet contents at high flowrates by chaotic advection [21]. In addition,a multi-step droplet splitting chip without passive mixing structures (winding channels) was fabricated for comparative experiments (Fig. 1a).
3.2. Influencing factors on the droplet splittingBesides channel dimension and flow condition,the interfacial tension of the fluids is an important factor influencing the droplet splitting.Weadjusted the interfacial tension by adding a surfactant (Span 80,2 wt%) into the continuous oil phase. Under viscous force and pressure force,the food dye solution and the mineral oil were firstly pushed towards the flow-focusing junction to form mother droplets. And then each of the mother droplets was broken into two daughter droplets via the first Y-shaped splitting junction and flowed down to branch channels. Such splitting process was repeated for three times,and eight lines of daughter droplets were finally generated. It was known that change of the flow rates and their ratio would lead to different states of water phase in the multiphase flow systems,e.g. laminar flow,plugs,cobbles and drops [23],and the aqueous droplets could be formed by adjusting two phase flowrates (the aqueous phase flow rate Qw and the oil phase flow rates Qo) and their ratio Q (Q = Qo/Qw). In the present work,three flow states were observed with constant Qw of 1 μL/ min: laminar flow with Q ≤ 0.4,droplets with 0.4 < Q < 30 and flow of oil phase alone with Q ≥ 30 (Fig. 1S in Supporting information).
The effect of different oil phase flowrates on the droplet splitting was investigated with constant Qw of 1 μL/min and 0.4 < Q < 30. To illustrate the whole splitting process visually,the images of droplets at different spots on the chip were taken as shown in Fig. 2. The mother droplets with yellow food dye solution were formed at the flow-focusing junction firstly,and then split equally into two daughter droplets at the first Y-shaped junction. After three times of splitting,the mother droplets were finally broken into 8 lines of small daughter droplets with one-eighth of the volume of mother droplets.

|
Download:
|
| Fig. 2.Images of droplets formed and split into smaller droplets using splitting junctions. (a) Mother droplet formation; (b) The first step division; (c) and (d) The second step division; (e) and (f) The third step division. The aqueous phase flowrate and the oil phase flowrate are both 1 μL/min. The scale bar is 500 μm. | |
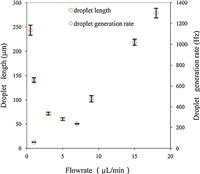
The effects of the flowrate on the droplet generation rate and the droplet size were investigated,as shown in Fig. 3. With the aqueous phase flowrate of 1.0 μL/min,the droplet generation rate was obviously augmented as the flowrate of oil phase varied from 1 μL/min to 18 μL/min. The droplet generation frequency of the whole chip was increased from 59 Hz to 1300 Hz. In the present multi-step droplet splitting system,the final droplet generation rate was increased to 8 times of the droplet generation rate in a single channel. It was demonstrated that the droplet generation rate could be sharply raised by simply adjusting the flowrate. Subsequently,to explore the dynamics of splitting,we measured the length of the droplets along their central axes under different oil phase flowrates. As expected,a significant decrease of the droplet length was observed with the increment of the oil phase flowrate from 0.5 μL/min to 3 μL/min (Fig. 3). During the droplet splitting process,the fluid resistance upstream will be influenced, which will lead to instability of the flowrate and irregularity of the droplet size. To ensure the stability of the flowrate,the branch channels downstream should keep a certain length to enhance the hydrodynamic resistance of channels. In this work,all of the branch channels were more than 4 mm. Thus the fluid resistance generated by the droplet splitting could be ignored,and stable droplet splitting was achieved in the range of oil phase flowrate from 0.5 μL/min to 5 μL/min using the chip with winding channels. The desired uniformity of the droplets was obtained. Relative standard deviation of the length of the droplets formed in a single channel kept within the range of 2%-5%,while in all the eight channels within the range of 3%-6%.
|
Download:
|
| Fig. 3.Effects of the oil phase flowrate on droplet length and droplet generation rate with aqueous phase flowrate of 1.0 μL/min. | |
At the beginning of the experiment,we tried to encapsulate single cells into the droplets using the chip without winding channels. The results showed that the cell encapsulations in parallel channels were inconsistent,as shown in Fig. 4. Most droplets were empty in one channel,while most droplets encapsulated multiple cells in the other channel. When the chip with winding channels was employed,the encapsulation of blood cells was obviously improved (Fig. 5). The number of droplets containing cells was nearly the same in the parallel channels. The reason is that the winding channels upstream the splitting junctions induced sufficient mixing of blood cells in droplets, and the dispersion of blood cells was markedly improved. The cells were divided into the daughter droplets with uniform probability distribution when the mother droplets were split. Therefore,the number of the droplets encapsulating blood cells was consistent in the parallel branch channels.

|
Download:
|
| Fig. 4.Images of the cell-encapsulating droplets in different channels of the chip without winding channels. The cell-encapsulating droplets are highlighted by red arrows. The aqueous phase flow rate is 0.2 μL/min and the oil phase flow rates is 0.5 μL/min. | |

|
Download:
|
| Fig. 5. Images of the cell-encapsulating droplets in parallel channels of the chip with winding channels. a-d, Images of droplets taken at four of eight outlets. The cellencapsulating droplets are highlighted by red arrows, the number of which was almost consistent in each image. The flowrates are same as in Fig. 4. | |
As the droplet splitting method is purely passive encapsulation based on hydrodynamic resistance,the concentration of cells in dispersed phase is one of the important factors determining the number of cells encapsulated in droplets. When the blood cell suspension solution was sheared into droplets,the cells would be evenly distributed into each of the droplets in theory. For investigating the number of cells encapsulated,the human whole blood was diluted,respectively,into cell suspensions with dilution ratio (Rd) being 1/10,1/50,1/100,1/200 and 1/500 by cell suspension medium. The results showed that the number of cells encapsulated in single droplets was more than ten with Rd ≥ 1/50, and less than four with 1/200 ≤ Rd ≤ 1/100. And most of the droplets were empty with Rd ≤ 1/500. The dispersed phase of human blood cell suspension with Rd of 1/200 was adopted to get the droplets encapsulating single cells. Besides,the density of cell suspension was investigated to improve the encapsulation of cells. As the blood cells tended to deposit on the bottom of the syringe and the tube under the effect of gravity,the density of cell suspension was adjusted by adding cell separating medium OptiPrepTM. The cell suspension containing 10% cell separating medium was used in this work for keeping the cell suspending. Finally,the human blood cell encapsulation was carried out using cell suspension with Rd of 1/200 (the theoretical blood cell concentration of 2.5 × 104 cells/mL),the aqueous phase flowrate of 0.2 μL/min and the oil phase flowrate of 0.5 μL/min. A single-cell encapsulation efficiency of 31% was obtained,and the multicellular droplet percentage was only 1.3%,as shown in Fig. 6. Compared with the single-cell encapsulation efficiency of 22% reported by Weitz group [11],the single-cell encapsulation was obviously improved.

|
Download:
|
| Fig. 6.Images of single-cell-encapsulating droplets using the chip with winding channels. The single-cell-bearing droplets are highlighted by red arrows, and the two-cell-bearing droplets are highlighted by red circles. The flowrates are same as in Fig. 4. | |
We developed a single-cell encapsulation method based on a microfluidic multi-step droplet splitting system. It was characterized by simple channel structure,high throughput of droplet production and high encapsulation efficiency. The high throughput of droplet production benefited from the multi-step splitting, which multiplied the productivity of droplets compared with the single droplet generator.Themonodisperse dropletswere generated with a throughput of more than one thousand Hz. The limited efficiency of passive encapsulation mode was promoted by improving the dispersed state of cell suspension by means of intensifying themixing of cells bywinding channels andovercoming the cell sedimentation by adding cell separating medium. The single-cell-encapsulating droplets reached 31%,and the multi-cellencapsulating droplets decreased to l.3%. This single-cell encapsulation technique has great potential for single cell analysis by integrating with other functional modules on the chip.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (Nos. 21305010,21375012),Fundamental Research Funds for the Central Universities (No. N140504002) and General Scientific Research Projects of Liaoning Provincial Department of Education (No. L2013106).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.10.016.
| [1] | M.E. Lidstrom, D.R. Meldrum, Life-on-a-chip, Nat. Rev. Microbiol. 1 (2003) 158-164. |
| [2] | A. Manz, N. Graber, H.M. Widmer, Miniaturized total chemical analysis systems: a novel concept for chemical sensing, Sens. Actuators, B: Chem. 1 (1990) 244-248. |
| [3] | R.N. Zare, S. Kim, Microfluidic platforms for single-cell analysis, Annu. Rev. Biomed. Eng. 12 (2010) 187-201. |
| [4] | Y. Liu, A.K. Singh, Microfluidic platforms for single-cell protein analysis, JALA 18 (2013) 446-454. |
| [5] | A.M. Thompson, A.L. Paguirigan, J.E. Kreutz, et al., Microfluidics for single-cell genetic analysis, Lab Chip 14 (2014) 3135-3142. |
| [6] | M.Y. He, J.S. Edgar, G.D.M. Jeffries, et al., Selective encapsulation of single cells and subcellular organelles into picoliter- and femtoliter-volume droplets, Anal. Chem. 77 (2005) 1539-1544. |
| [7] | S.K. Fan, P.W. Huang, T.T. Wang, et al., Cross-scale electric manipulations of cells and droplets by frequency-modulated dielectrophoresis and electrowetting, Lab Chip 8 (2008) 1325-1331. |
| [8] | U. Demirci, G. Montesano, Single cell epitaxy by acoustic picolitre droplets, Lab Chip 7 (2007) 1139-1145. |
| [9] | Y. Zeng, R. Novak, J. Shuga, et al., High-performance single cell genetic analysis using microfluidic emulsion generator arrays, Anal. Chem. 82 (2010) 3183-3190. |
| [10] | S.Q. Gu, Y.X. Zhang, Y. Zhu, et al., Multifunctional picoliter droplet manipulation platform and its application in single cell analysis, Anal. Chem. 83 (2011) 7570-7576. |
| [11] | S. Koester, F.E. Angile, H. Duan, et al., Drop-based microfluidic devices for encapsulation of single cells, Lab Chip 8 (2008) 1110-1115. |
| [12] | E. Um, S.G. Lee, J.K. Park, Random breakup of microdroplets for single-cell encapsulation, Appl. Phys. Lett. 97 (2010) 153703-1-153703-3. |
| [13] | M. Chabert, J.L. Viovy, Microfluidic high-throughput encapsulation and hydrodynamic self-sorting of single cells, Proc. Natl. Acad. Sci. U.S.A. 105 (2008) 3191-3196. |
| [14] | J. Clausell-Tormos, D. Lieber, J.C. Baret, et al., Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms, Chem. Biol. 15 (2008) 427-437. |
| [15] | J.F. Edd, D. Di Carlo, K.J. Humphry, et al., Controlled encapsulation of single-cells into monodisperse picolitre drops, Lab Chip 8 (2008) 1262-1264. |
| [16] | E.W.M. Kemna, R.M. Schoeman, F. Wolbers, et al., High-yield cell ordering and deterministic cell-in-droplet encapsulation using Dean flow in a curved microchannel, Lab Chip 12 (2012) 2881-2887. |
| [17] | D.R. Link, S.L. Anna, D.A. Weitz, et al., Geometrically mediated breakup of drops in microfluidic devices, Phys. Rev. Lett. 92 (2004) 054503-1-054503-4. |
| [18] | C.G. Yang, Z.R. Xu, A.P. Lee, et al., A microfluidic concentration-gradient droplet array generator for the production of multi-color nanoparticles, Lab Chip 13 (2013) 2815-2820. |
| [19] | A.R. Abate, D.A. Weitz, Faster multiple emulsification with drop splitting, Lab Chip 11 (2011) 1911-1915. |
| [20] | J.C. McDonald, D.C. Duffy, J.R. Anderson, et al., Fabrication of microfluidic systems in poly(dimethylsiloxane), Electrophoresis 21 (2000) 27-40. |
| [21] | H. Song, J.D. Tice, R.F. Ismagilov, A microfluidic system for controlling reaction networks in time, Angew. Chem. Int. Ed. 42 (2003) 768-772. |
| [22] | D. Di Carlo, D. Irimia, R.G. Tompkins, et al., Continuous inertial focusing, ordering, and separation of particles in microchannels, Proc. Natl. Acad. Sci. U.S.A. 104 (2007) 18892-18897. |
| [23] | C.G. Yang, Z.R. Xu, J.H. Wang, Manipulation of droplets in microfluidic systems, Trac-trend. Anal. Chem. 29 (2010) 141-157. |
 2015, Vol.26
2015, Vol.26 


