Today,there is an increasing demand for chemical sensors to detect a variety of analytes for a range of applications,including environment monitoring,homeland security guarding,and food quality controlling. Typically,chemical sensors can bind selectively and reversibly the analytes of interest with a concomitant change in electrical,optical,or mechanical properties. Among all the chemosensors,luminescence-based ones present many advantages in terms of definite fluorescence spectrum,visible signal by naked eye,high sensitivity and selectivity,and ease of operation. Even so,there still remain great challenges to realize better sensory performance including increased brightness,higher quantum yield,excellent photo-stability under UV irradiation,and multifunctional systems. Here,a particular class of materials wellknown as metal-organic frameworks (MOFs) will be presented to construct a robust platform to deal with these challenges.
MOFs,a promising class of hydrid materials constructed from straight self-assembly of metal cations/clusters and organic ligands through coordination bonds,have been extensively studied over the past two decades. The variety of metal and organic ligand building units makes it possible to prepare virtually unlimited MOFs to utilize many potential applications in numerous areas, including gas adsorption [1],separation [2, 3],catalysis [4, 5], chemical sensing [6, 7, 8],opto-electronics [9],clean energy [10], bio-imaging and drug delivery [11]. Among them,MOFs are utilized for chemical sensing mostly based on the fluorescence emission [12],because all of metal ions,organic ligands,metal- organic charge transfer,and luminescent guest molecules can generate luminescence [13, 14]. And corresponding changes act in response to the uptake of analyte guests,as a mean of signal transduction. Furthermore,the functional sites in MOFs such as Lewis basic/acidic sites and unsaturated metal sites can be used for selective recognition of targeted molecules/ions. The tunable porosity can enable the reversible storage of guest species,making MOFs both as detection and pre-concentrator medium.
However,MOFs in the common form of bulk crystals cannot meet the specific requirements for analytical applications. In view of this,MOF materials are needed to be miniaturized at a nanometer scale or immobilized on selected substrates for practical applications. Nanoscale MOFs (NMOFs) have been proved to be a kind of effective sensory materials [15]. First,NMOF materials exhibit not only the rich diversity of compositions, structures,and properties as the bulk MOFs,but also the high dispersity in aqueous solution and the intrinsic biocompatibility, which facilitate the wide applications of NMOFs in the fields of chemical sensing [16, 17]. Second,NMOF materials display extremely high surface areas to enhance the sensory performance in both response time and detection sensitivity due to a preconcentration effect,so the analytes are absorbed and concentrated inside the NMOF channels. The combination of nanoscale processability,intrinsic luminescent properties,and permanent porosity of NMOFs will definitely create a bright prospect for designing novel luminescent sensory nanomaterials with enhanced desired multifunctionalities.
2. Syntheses of luminescent nanoscale MOFsLike macroscopic counterparts,NMOFs are mainly prepared through the "bottom-up" synthetic strategy based on straight selfassembly of inorganic and organic components. The synthesis of nanoscale metal-organic materials and porous materials has recently been reviewed [17, 18],so general synthetic strategies for luminescent NMOFs are outlined. In order to clearly interpret various synthetic methods,these NMOF materials are classified into three broad classes: class I,direct luminescent MOF nanoparticles; class II,host NMOFs containing luminescent guests; class III,2-dimension (2-D) NMOF luminescent films.
2.1. Direct luminescent MOF nanoparticlesThree different strategies are generally applied for synthesizing MOF nanoparticles: (i) the nano-precipitation method; (ii) the confinement at nanoscopic regime by using emulsions or templates; (iii) microwave or ultrasound assisted synthesis.
First of all,nano-precipitation is the simplest method for the preparation of MOF nanoparticles. This method usually involves two steps: (i) the dissolution of reaction precursors in a good solvent,(ii) the addition of a poor solvent to stop the assembly process,resulting in the formation of particles at a nanometer scale. Mirkin and Wang have done pioneering work to develop this methodology [19, 20]. Lin applied this strategy to synthesize MOF nanoparticles constructed from the anticancer drug c,c,t-(diamminedichlorodisuccinato) Pt(IV) (DSCP) and Tb3+ ions [21]. Typically, a precursor solution of TbCl3 (15 mmol) and DSCP (10 mmol) was mixed in H2O,and the pH value was adjusted to 5.5. After that, methanol was rapidly added with vigorous magnetic stirring, which resulted in the formation of nanoparticles. However,this nano-precipitation method is not appropriate for crystalline NMOFs.
The second family of strategies for miniaturizing MOFs is the nanoscopic confinement effect assisted by surfactant. Lin developed a reverse microemulsion methodology to synthesize NMOFs, such as Gd(BDC)1.5(H2O)2,[Gd(BTC)(H2O)3]·H2O [22],and Gd2(BHC)(H2O)6 [23]. Reverse microemulsions were formed by using surfactants to stabilize water droplets within a nonpolar organic phase. Two separate microemulsions containing either Ln3+ ions or organic linkers were mixed and reacted for a given period of time to form crystalline MOF nanoparticles. Eu3+ and Tb3+-doped Gd(BDC)1.5(H2O)2 nanorods were also prepared and proved to be luminescent.
The third family of strategies for miniaturizing MOFs is microwave or ultrasound assisted synthesis. Generally speaking, microwave irradiation can accelerate the crystal nucleation and produce nanocrystals with narrower size distributions than conventional solvothermal approach. Nanoscale Tb-MOF-76 was prepared through a rapid microwave-assisted method with proline and glycine as capping agents [24]. It exhibited a typical emission of Tb3+ ions,which was sensitive to acetone in aqueous solution. Similarly,ultrasonic irradiation can generate a homogenous nucleation and a substantial reduction in crystallization time. Well-defined Mg-MOF-74 nanocrystals with diameters of 100 nm were successfully synthesized in one hour in the presence of triethylamine as a deprotonating agent [25].
As for conventional solvothermal synthesis,various additives have been proved to suppress NMOF growth through a coordination modulation route. First,alkylamine additives such as triethylamine [25],n-butylamine and diethylamine [26] effectively deprotonate the metal complex and accelerate crystal nucleation, resulting in an obvious decrease of crystal sizes. Second, carboxylate and N-heterocycle additives,such as benzoic acid [27], acetate acid [28],sodium formate,sodium acetate [29],and 1- methylimidazole [30],have the same chemical functionality as the bridging linkers to impede the coordination interaction between the metal ions and the bridging linkers. Luminescent Eu1-xTbx- MOFs nanocrystals around 100 nm were synthesized with the addition of sodium formate or sodium acetate,and further fabricated to form continuous films via spin-coating deposition [29]. The films exhibited strong luminescent properties and thus showed potential applications in the field of luminescent sensors. Other additives such as p-perfluoroethylbenzoic acid [31], dodecanoic acid [32],4n-decylbenzoic acid [33] and polymers such as poly(acrylic acid sodium salt) [34] and poly(diallyldimethylammonium chloride) [35] were also used to control the sizes and morphologies of NMOFs.
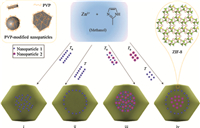
2.2. Host NMOFs containing luminescent guestsConfinement of different guests,especially luminescent guests, into pores of NMOFs makes them versatile candidates to develop multifunctional sensory materials. One of the ideal methods is the direct incorporation of luminescent guests during the process of NMOF synthesis. Maspoch prepared metal-organic nanospheres constructed from Zn2+ and 1,4-bis(imidazol-1-ylmethyl)benzene (bix) by a fast precipitation [36]. These Zn(bix) nanospheres were used as functional matrices for the encapsulation of a variety of substances,such as luminescent quantum dots (QDs),fluorescein, rhodamine B,or two kinds of substances. The formed nanoparticles retained the intrinsic luminescent properties of guest species. Recently,a great breakthrough was the introduction of polyvinylpyrrolidone (PVP) as capping agents into the synthetic system of NP@MOF core-shell nanostructures [37]. A variety of PVPcapped nanoparticles (NP) was successively adsorbed on the continuously forming surfaces of ZIF-8 nanocrystals to form NP/ ZIF-8 hybrid nanocrystals (Fig. 1). Imparting luminescent properties to ZIF-8 nanocrystals was demonstrated through separate encapsulation of lanthanide-doped NaYF4 nanorods and CdTe QDs. Remarkably,the luminescent features of different cores were well maintained. Chi reported a highly-luminescent NMOF material through encapsulating the branched poly-(ethylenimine)-capped carbon quantum dots into ZIF-8 [38].
|
Download:
|
| Fig. 1.Schematic presentation of the controlled encapsulation of various PVPcapped nanoparticles into ZIF-8 nanocrystals. Reprinted with permission from Ref. [37], Copyright 2012, Nature Publishing Group. | |
Postsynthetic loading strategy,usually ion-exchange method,is another effective method to introduce luminescent ions or molecules into NMOF pores. For example,different lanthanide cations were introduced into cationic bio-MOF-1 pores through soaking bio-MOF-1 samples in DMF solution of Tb3+,Sm3+,Eu3+, Yb3+ salts,respectively [39]. The resulted Tb@bio-MOF-1,Sm@bio- MOF-1,and Eu@bio-MOF-1 samples emitted their distinctive colors: green,orange-pink,and red,respectively. The resulted Yb@bio-MOF-1 emitted NIR luminescence and was used as oxygen sensors. Besides lanthanide cations,pyridinium hemicyanine dye was encapsulated into bio-MOF-1 pores to realize a new synergistic two-photon-pumped lasing functionality [40].
2.3. 2-D NMOF luminescent filmsLuminescent NMOFs can be fabricated into thin films for their straightforward sensing applications. So far,the general strategy is the direct nucleation and growth of MOFs on selected substrates, e.g. SiO2,Al2 O3,Au,etc [41, 42]. However,the bare substrate surfaces usually inhibited the nucleation of NMOFs. Caro [43],Qiu [44] and Ameloot [45] reported the chemical modification of substrates through the oxidation of substrate surfaces to create more surface nucleation points to obtain dense coatings. In addition,the addition of crystal seeds is another proposed way to induce a better crystallization process to fabricate robust NMOF membranes [46, 47].
Recently,Terfort developed an effective ink-jet printing technology to realize the controlled deposition of HKUST-1 onto plastics,papers,and textiles (Fig. 2) [48]. The "ink" was a precursor solution of Cu(NO3)2·3H2O and BTC in mixed solvents of dimethyl sulfoxide,ethanol,and ethylene glycol. The desired patterns were simply obtained by printing-drying process and the thickness was directly controlled by multiple "printing-drying" cycles. Textiles with ink-printed HKUST-1 could detect several toxic gases (NH3, H2S,HCl, etc.) through a unique color change.

|
Download:
|
| Fig. 2.(a) The applicability of HKUST-1 for large area patterning; (b) various graphic patterns printed onto PET foil; (c) SEM images of printed HKUST-1 after 8 printingdrying cycles; (d) adsorption capacities of HKUST-1 films on QCM for various gases. Reprinted with permission from Ref. [48], Copyright 2013, Wiley-VCH. | |
The luminescent properties of NMOFs are very sensitive to subtle structural changes including coordination environment of metal ions,interactions between host framework and guest species,porosity,and energy transfer between ligands and metal centers. Therefore,the stimuli-responsive properties of NMOFs make it an ideal candidate working as chemosensory materials. Over the past few years,luminescent NMOF materials have been reported to detect ion species,organic molecules,noxious gases, explosives,pH value,and temperature.
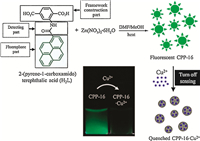
3.1. Detection of ion species 3.1.1. Detection of Cu2+ ionsRational design of MOF with special sites to selectively bind analytes is of significant importance for sensing applications. Oh developed an excellent strategy to design a chemosensory particle, as named CPP-16,consisting of a crystalline framework,a fluorophore part,and a Cu2+ detecting part (Fig. 3) [49]. CPP-16 was fluorescent due to the incorporation of fluorescent pyrenefunctionalized building blocks. With the addition of Cu2+,the luminescence intensity of CPP-16 decreased. The detection mechanism was attributed to the specific interaction between Cu2+ and amide group of CPP-16,causing pyrene moieties to undergo conformation changes from the stacked excimer state to the quenched excimer state.
|
Download:
|
| Fig. 3.Schematic representation of CPP-16 as a "turn-off" fluorescent chemosensor to detect Cu2+ ion. Reprinted with permission from Ref. [49], Copyright 2014, American Chemical Society. | |
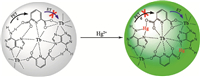
By using thiol-functionalized 2,5-dimercapto-1,4-benzenedicarboxylic acid (H2DMBD) as the organic linker,a Zr-DMBD MOF with size of 200 nm was prepared [50]. Zr-DMBD was capable of effective uptake of Hg2+ ions from aqueous solution and gaseous phase,because of the strong binding between thiol groups and Hg2+. After mercury uptake,the emission at 500 nm was largely suppressed and hardly visible to naked eye. Chen prepared coordination polymer nanoparticles composing of adenine (Ad), Tb3+ ion,and dipicolinic acid (DPA) (Fig. 4) [51]. The DPA molecule was expected to sensitize the fluorescence of Tb3+. However,the fluorescence of nanoparticles was very weak because intramolecular energy transfer (ET) from DPA to Tb3+ was impeded by photoinduced electron transfer (PET) from adenine to DPA. When Hg2+ was added,a significant enhancement in emission intensity was observed because of the suppression of PET process through the coordination between Hg2+ with adenine. These nanoparticles exhibited excellent selectivity and ultra-high sensitivity up to the 0.2 nM detection limit.
|
Download:
|
| Fig. 4.Schematic illustration of Ad/Tb/DPA nanoparticles for sensing of Hg2+ ions. Reprinted with permission from Ref. [51], Copyright 2012, American Chemical Society. | |
Yan reported the exploration of luminescent nMOF-253 nanomaterial for highly selective and sensitive detection of Fe2+ ions in aqueous solution [52]. The detection mechanism was likely attributed to PET process and the coordination between Fe2+ and bipyridine. Moreover,nMOF-253 was successfully applied in luminescent bioimaging and intracellular Fe2+ sensing in HeLa cells.
3.1.4. Detection of Fe3+ ionsLuminescent MOF nanocrystals were prepared through encapsulating Eu3+ ions into MIL-53-COOH (Al) pores,showing highly selective and sensitive detection of Fe3+ in aqueous solution [53]. The possible sensing mechanism was based on ion exchange between the analyte Fe3+ and the framework Al3+.
3.2. Detection of organic molecules 3.2.1. Detection of organic solventsSolvatochromism is the ability of chemosensory material to change its color in response to a change of solvent polarity. For example,{[(WS4Cu4)I2(dptz)3]·DMF}n (dptz = 3,6-di(pyridin-4-yl)- 1,2,4,5-tetrazine) MOF displayed different colors after immersed in solventswith different polarity [54]. Kitagawa proposed amolecular decoding methodology to prove that [Zn2(bdc)2(dpNDI)]n (dpNDI = N,N'-di(4-pyridyl)-1,4,5,8-naphthalenediimide) MOF distinguished different guests confined in the nanopores by transducing a particular host-guest interaction into a corresponding color change [55]. And {[Tb2(NO3)2(edc)(DMF)4]·DMF·H2O}n with minimal diameters of 70 nm acted as a luminescent probe to recognize cyclohexane and nitrobenzene with high sensitivity and quick regeneration ability [56].
3.2.2. Detection of dipicolinic acidDipicolinic acid (DPA) is an important molecular marker in spore producing bacteria,which constitutes up to 15% of dry mass of spores. Lin developed a novel method to functionalize silicacoated NMOFs for the luminescence sensing of DPA [57]. Eu-doped Gd(bdc)1.5(H2O)2 nanoparticles were coated orderly with PVP molecules,silica nanoshell,and silylated Tb-EDTM monoamide derivative to form silica-coated NMOFs. Under excitation at 278 nm,the composite only gave Eu3+ luminescence because Tb-EDTM was essentially non-emissive. When DPA was added,the Tb3+ luminescence became clearly visible due to the formation of Tb-EDTM-DPA complex. Therefore,the Tb3+ emission provided a sensitive probe for DPA,while the Eu3+ emission in the NMOF core acted as a non-interfering internal calibration. Afterward,Chen and Qian reported Eu2(FMA)2(OX)(H2O)4·4H2O (FMA = fumarate, OX = oxalate) of about 200-400 nm,whose size and morphology was simply tailored by the addition of different amount of CTAB [58]. The as-made NMOF exhibited highly sensitive,selective and instant "turn-on" sensing of DPA,because the coordination between DPA molecules and Eu3+ significantly enforced the intramolecular energy transfer.
3.2.3. Detection of explosive moleculesChemical sensors for rapid detection of explosives are attracting many attentions due to homeland security,anti-terrorism,and humanitarian implications. Luminescent NMOF materials have been utilized for effective detection of high explosive molecules. Chen and Qian employed the water-in-oil microemulsion strategy to synthesize Eu2(BDC)3(H2O)2(H2O)2 nanorods of less than 100 nm [59]. The ethanol dispersion of MOF nanorods exhibited typical emission of Eu3+ ions. The nitroaromatic compounds such as 2,4-dinitrotoluene (DNT) and 2,4,6-trinitrotoluene (TNT) showed a significant quenching effect on the emission intensity of the NMOF dispersion,maybe because of a competitive absorption of the light source energy and the electronic interaction between the nitroaromatic compounds and ligands. Zang reported a Zn-bcpa NMOF with cubic shape of 500-1000 nm in size (bcpa = 9,10-bis(p-carboxyphenyl)anthracene) [60]. Its fluorescence emission was quenched upon interaction with the nitroaromatic explosives (e.g. DNT,TNT) and nitroaliphatic explosives (e.g. nitromethane). Even when the concentration of nitromethane was diluted down to 1% of the saturated vapor,the emission was still quenched. The detection limit of nitromethane was down to ppm level. The quenching mechanism was likely attributed to the PET process from the excited NMOFs to the adsorbed explosive molecules. Qiu used a self-sacrificing template strategy to synthesize luminescent CdBTC-MOF nanotubes as efficient chemical sensors for trace-level detection of DNT vapor [61].
3.3. Gas sensorsGas sensors have a wide range of applications in the fields of aerodynamics,environmental monitoring,analytical chemistry, and biochemistry. Luminescent NMOFs as gas sensors have been exploited because the luminescent properties of NMOFs can be perturbed by toxic gases and vapors.
3.3.1. Detection of H2SLu has fabricated a novel type of highly luminescent Cu(I)-NCPs through a nano-precipitation method [62]. These Cu(I)-NCPs have provided a rapid and sensitive platform to detect H2S vapor because H2S selectively quenched Cu(I)-NCPs’ fluorescence. Moreover,Cu(I)-NCPs served as "inks" for writeable detection of H2S. Chen reported a coordination polymer nanoparticle composing of Tb3+ ions,Ag+ ions,and adenosine monophosphate,where the fluorescence of Tb3+ ions was sensitized by Ag+ ions [63]. When H2S was introduced,a fluorescence quenching would be observed because of strong affinity of H2S to Ag+ ions.
3.3.2. Detection of O2Highly-sensitive sensors for trace oxygen analytes are demanded in many oxygen-free environments,such as medical environment,chemical industry,and so on. Some MOF materials with the incorporation of phosphorescent Ru/Ir-complexes have shown high oxygen-sensing efficiencies [64, 65]. However,bulk MOF crystals cannot be directly used as gas sensors,unless they are immobilized into solid devices or membranes. Chen synthesized a Cu(I)-triazolate [Cu(detz)] (Hdetz = 3,5-diethyl-1,2,4-trizole) NMOF,which was not luminescent in air due to the quenching effect by oxygen [66]. Indeed,Cu(detz) in vacuum showed luminescence,indicating that Cu(detz) exhibited O2-sensing properties. In order to realize its practical application,Cu(detz) was composited with silicon rubbers to fabricate soft and robust membranes by a counter-diffusion crystal-growth method. The membrane not only maintained original luminescence and oxygen-sensing properties,but also further improved the chemical stability of the Cu(detz) NMOF.
3.3.3. Detection of other gasesHKUST-1 framework contains a high density of unsaturated acid sites which can coordinate small gas molecules. When HKUST- 1 was printed on textiles,the textiles showed potential applications to capture hazardous gases [48]. Meanwhile,HKUST-1 rapidly changed its colors from turquoise to dark blue,yellow,and brown after the exposure to NH3,HCl,and H2S vapors,respectively. This color-change phenomenon,not only indicated the capture process,but also presented the possibility of inkjet-printed HKUST-1 to fabricate practical gas sensors.
3.4. pH sensorsOne of the significant subjects in MOF-based chemical sensors is the detection of pH values in aqueous solution,especially for monitoring subtle pH changes in biological environments and living cells. Yan exploited a post-synthetic method to simultaneously introduce two types of Eu3+ ions with different excitation wavelengths into nanoscale MOF-253 [67]. One of Eu3+ was sensitive to pH changes while the other was not,resulting in a ratiometric pH response in the pH range of 5.0-7.2. Recently,a novel type of NMOF based pH sensors has been explored in realtime sensing and monitoring of intracellular pH changes inside live cells [68]. Fluorescein isothiocyanate (FITC) is pH sensitive and exhibits pH-dependent ratiometric fluorescence changes. However, the utility of FITC in live cell imaging is severely limited by its rapid release from cells. In order to address the challenges,Lin designed a novel NMOF sensor (called F-UiO) through covalent linkage of FITC to amino-modified UiO-MOF (Fig. 5). The F-UiO nanocomposites with a diameter of 100 nm and a thickness of 30 nm exhibited structural stability,high fluorescent efficiency,pH response sensitivity,and efficient cellular uptake. The ratiometric pH-sensitivity of F-UiO was determined with pH value range of 4.0-8.0 by a fluorometer. The ratios of 520 nm emission intensity under excitation at 488 and 435 nm (I488/520/I435/520) were calculated,and the correlation of emission intensity ratio to pH was established by nonlinear curve fitting. Upon rapid and efficient endocytosis,F-UiO maintained the structural integrity inside endosomes. Live cell imaging was performed to monitor the endocytosis of F-UiO and endosome acidification process in real time.

|
Download:
|
| Fig. 5.(Left) (a) Schematic illustration of F-UiO synthesis; (b) PXRD patterns of UiO, F-UiO, and F-UiO after incubating in HBSS for 12 h; (c) TEM images of F-UiO; and (d) F-UiO after incubating in HBSS for 12 h. (Right) (a) Correlation between FITC absorbance and fluorescence at various FITC loadings; (b–d) pH calibration curves of free FITC (b) and FUiO acquired by fluorimetry (c) and by CLSM (d). (e) CLSM images showing the colors of F-UiO particles in HBSS buffers with different pH values. Reprinted with permission from Ref. [68], Copyright 2014, American Chemical Society. | |
The mixed-lanthanide (usually Eu3+ and Tb3+ ions) MOF materials show unique temperature-dependent luminescent properties,giving rise to their potential applications as luminescent thermometers. In 2010,Palacio and Carlos have done the pioneering work to develop a unique Eu3+/Tb3+ luminescent nanothermometer [69]. The thermometer consisted of [Eu(btfa)3(- MeOH)(bpeta)] and [Tb(btfa)3(MeOH)(bpeta)] β-diketonate chelates (btfa = 4,4,4-trifluoro-l-phenyl-1,3-butanedione,bpeta = 1,2- bis(4-pyridyl)ethane),which were embedded into organic-inorganic hybrid nanoclusters formed by a magnetic core and a TEOS/ APTES organosilica shell. As the temperature in the range of 10- 350 K increased,the emission intensity of Tb3+ strongly decreased, while that of Eu3+ started to increase. The mechanism was assumed to be a two-steps process of Tb3+→host→Eu3+ energy transfer. Although the thermometer showed temperature sensitivity up to 4.9% per K,the synthesis procedure was complicated and two-steps energy transferwas obviously inefficient. Qian and Chen have improved this strategy to fabricate luminescent Ln-MOF thermometers,which was based on direct energy transfer from Tb3+ to Eu3+ within the same framework [70]. They designed an organic ligand H2DMBDC to sensitize both Eu3+ and Tb3+ emissions and constructed a mixed-lanthanide MOF (EuxTb1-x)2(DMBDC)3(H2O)4·DMF·H2O,showing two characteristic emissions of Tb3+ ions at 545 nm and Eu3+ ions at 613 nm,as expected. Taking x = 0.0069 as an example,at 10 K the emission peaks of 545 nm (Tb3+) and 613 nm (Eu3+) exhibited comparable intensity. However,when the temperature was increasing,the emission intensity of the Tb3+ ions decreased,while that of the Eu3+ increased. At 300 K,the emission band was almost at 613 nm and that at 545 nm was nearly invisible. The unique behavior suggested an efficient energy transfer from Tb3+ to Eu3+ with increasing temperature. Furthermore,the emission intensity ratio (ITb/IEu)was linearly related to the temperature in awide range from 10 K to 300 K,highlighting an ideal luminescent thermometer.
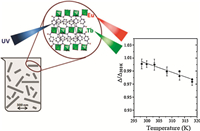
Afterwards,Rocha and Carlos have prepared nanorods of Tb0.99Eu0.01(BDC)1.5·(H2O)2 through a reversemicroemulsion method (Fig. 6) [71]. The resulted nanorods in an aqueous suspension displayed an excellent performance as ratiometric luminescent nanothermometers in the physiological temperature range of 300-320 K. The temperature-dependence of the 5D0→7F2 (Eu3+) and 5D4→7F5 (Tb3+) energy transfer was used to define the ratiometric thermometric parameter. Recently,they prepared [(Tb0.914Eu0.086)2(PDA)3(H2O)]·2H2O (PDA = 1,4-phenylenediacetic acid) nanoparticles by the spray-drying method. It is an excellent nano-thermometer in the range of 10-325 K with high sensitivity (up to 5.96 ± 0.04% K-1 at 25 K),high reproducibility (>97%,at room temperature),and low-temperature uncertainty (0.02 K at 25 K) [72].
|
Download:
|
| Fig. 6.Schematic illustration of luminescent Tb0.99Eu0.01(BDC)1.5(H2O)2 nanorods as a ratiometric nanothermometer in the physiological temperature range of 300– 320 K. Reprinted with permission from Ref. [71], Copyright 2013, American Chemical Society. | |
Luminescent NMOFs are an exactly fascinating class of multifunctional chemosensors through the rational design of NMOF materials with tailorable particle sizes,suitable porosity, specific functional groups,strong electron and energy transfer,and tunable luminescent properties. Although at the early stage of their development,luminescent NMOFs have been a versatile and remarkable type of chemical sensors for the detection of ions, molecules,gases,pH value,and temperature.
These achievements are just a tip of iceberg and there are still many challenges in the area of chemical detection. First of all,a major challenge is to pre-design novelNMOFmaterials with desired properties. Any specific application can theoretically be realized through the rational design of NMOF materials in the terms of compositions,sizes,shapes,nanoporosity,and luminescent properties. Second,the majority of NMOF sensors are based on the monitoring of emission intensity changes in a single emission peak by a fluorometer. The accuracy can be heavily affected by the optoelectronic drifts of excitation power and detectors. Ratiometric or self-calibrated chemosensory materials can avoid the drawbacks of those based on single-emission intensity,making precise chemical detection possible. Third,the targeted analytes are limited to a few types of guests,such as strong electron receptors or donors. An improved approach is to couple luminescent NMOFs with other methods of signal transduction,such as interferometry,localized surface plasmon resonance (LSPR),surface-enhanced Raman spectroscopy (SERS),and electrical/electrochemical/mechanical schemes,developing multifunctional NMOFs for the diverse chemosensory applications. Forth,other synthetic methodologies (e.g. patterned deposition [73],microfluidics [74/span>],supercritical conditions,etc.) and lithographic techniques (e.g. photolithographic, dip-pen nanolithographic [75],fountain-pen lithographic,etc.) can be implemented to expand the variety of synthesized NMOFs. Briefly,nanoscale luminescentMOFs will be extensively pursued for their practical applications in chemical detection.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (No. 21303178) and Jilin Province Youth Foundation (No. 20140520091JH).
| [1] | J.R. Li, R.J. Kuppler, H.C. Zhou, Selective gas adsorption and separation in metal- organic frameworks, Chem. Soc. Rev. 38 (2009) 1477-1504. |
| [2] | J.R. Li, J. Sculley, H.C. Zhou, Metal-organic frameworks for separations, Chem. Rev. 112 (2012) 869-932. |
| [3] | D. Liu, J.P. Lang, B.F. Abrahams, Highly efficient separation of a solid mixture of naphthalene and anthracene by a reusable porous metal-organic framework through a single-crystal-to-single-crystal transformation, J. Am. Chem. Soc. 133 (2011) 11042-11045. |
| [4] | J.Y. Lee, O.K. Farha, J. Roberts, et al., Metal-organic framework materials as catalysts, Chem. Soc. Rev. 38 (2009) 1450-1459. |
| [5] | D. Liu, Z.G. Ren, H.X. Li, et al., Single-crystal-to-single-crystal transformations of two three-dimensional coordination polymers through regioselective [2 + 2] photodimerization reactions, Angew. Chem. Int. Ed. 49 (2010) 4767-4770. |
| [6] | B.L. Chen, S.C. Xiang, G.D. Qian, Metal-organic frameworks with functional pores for recognition of small molecules, Acc. Chem. Res. 43 (2010) 1115-1124. |
| [7] | L.E. Kreno, K. Leong, O.K. Farha, et al., Metal-organic framework materials as chemical sensors, Chem. Rev. 112 (2012) 1105-1125. |
| [8] | M.M. Chen, X. Zhou, H.X. Li, X.X. Yang, J.P. Lang, Luminescent two-dimensional coordination polymer for selective and recyclable sensing of nitroaromatic compounds with high sensitivity in water, Cryst. Growth Des. 15 (2015) 2753-2760. |
| [9] | V. Stavila, A.A. Talin, M.D. Allendorf, MOF-based electronic and opto-electronic devices, Chem. Soc. Rev. 43 (2014) 5994-6010. |
| [10] | S.L. Li, Q. Xu, Metal-organic frameworks as platforms for clean energy, Energy Environ. Sci. 6 (2013) 1656-1683. |
| [11] | J.D. Rocca, D.M. Liu, W.B. Lin, Nanoscale metal-organic frameworks for biomedical imaging and drug delivery, Acc. Chem. Res. 44 (2011) 957-968. |
| [12] | Z.C. Hu, B.J. Deibert, J. Li, Luminescent metal-organic frameworks for chemical sensing and explosive detection, Chem. Soc. Rev. 43 (2014) 5815-5840. |
| [13] | M.D. Allendorf, C.A. Bauer, R.K. Bhakta, R.J.T. Houk, Luminescent metal-organic frameworks, Chem. Soc. Rev. 38 (2009) 1330-1352. |
| [14] | Y.J. Cui, Y.F. Yue, G.D. Qian, B.L. Chen, Luminescent functional metal-organic frameworks, Chem. Rev. 112 (2012) 1126-1162. |
| [15] | A.M. Spokoyny, D. Kim, A. Sumrein, C.A. Mirkin, Infinite coordination polymer nano- and microparticle structures, Chem. Soc. Rev. 38 (2009) 1218-1227. |
| [16] | A. Carné, C. Carbonell, I. Imaz, D. Maspoch, Nanoscale metal-organic materials, Chem. Soc. Rev. 40 (2011) 291-305. |
| [17] | M. Sindoro, N. Yanai, A.Y. Jee, S. Granick, Colloidal-sized metal-organic frameworks: synthesis and applications, Acc. Chem. Res. 47 (2014) 459-469. |
| [18] | V. Valtchev, L. Tosheva, Porous nanosized particles: preparation, properties, and applications, Chem. Rev. 113 (2013) 6734-6760. |
| [19] | M. Oh, C.A. Mirkin, Chemically tailorable colloidal particles from infinite coordination polymers, Nature 438 (2005) 651-654. |
| [20] | X.P. Sun, S.J. Dong, E.K. Wang, Coordination-induced formation of submicrometer- scale, monodisperse, spherical colloids of organic-inorganic hybrid materials at room temperature, J. Am. Chem. Soc. 127 (2005) 13102-13103. |
| [21] | W.J. Rieter, K.M. Pott, K.M.L. Taylor, W.B. Lin, Nanoscale coordination polymers for platinum-based anticancer drug delivery, J. Am. Chem. Soc. 130 (2008) 11584- 11585. |
| [22] | W.J. Rieter, K.M.L. Taylor, H.Y. An, W.L. Lin, W.B. Lin, Nanoscale metal-organic frameworks as potential multimodal contrast enhancing agents, J. Am. Chem. Soc. 128 (2006) 9024-9025. |
| [23] | K.M.L. Taylor, A. Jin, W.B. Lin, Surfactant-assisted synthesis of nanoscale gadolinium metal-organic frameworks for potential multimodal imaging, Angew. Chem. Int. Ed. 47 (2008) 7722-7725. |
| [24] | W.T. Yang, J. Feng, S.Y. Song, H.J. Zhang, Microwave-assisted modular fabrication of nanoscale luminescent metal-organic framework for molecular sensing, ChemPhysChem 13 (2012) 2734-2738. |
| [25] | T.H. Bae, J.R. Long, CO2/N2 separations with mixed-matrix membranes containing Mg2(dobdc) nanocrystals, Energy Environ. Sci. 6 (2013) 3565-3569. |
| [26] | Y.S. Li, H. Bux, A. Feldhoff, et al., Controllable synthesis of metal-organic frameworks: from MOF nanorods to oriented MOF membranes, Adv. Mater. 22 (2010) 3322-3326. |
| [27] | A. Schaate, P. Roy, A. Godt, et al., Modulated synthesis of Zr-based metal-organic frameworks: from nano to single crystals, Chem. Eur. J. 17 (2011) 6643-6651. |
| [28] | T. Tsuruoka, S. Furukawa, Y. Takashima, et al., Nanoporous nanorods fabricated by coordination modulation and oriented attachment growth, Angew. Chem. Int. Ed. 48 (2009) 4739-4743. |
| [29] | H.L. Guo, Y.Z. Zhu, S.L. Qiu, J.A. Lercher, H.J. Zhang, Coordination modulation induced synthesis of nanoscale Eu1-xTbx-metal-organic frameworks for luminescent thin films, Adv. Mater. 22 (2010) 4190-4192. |
| [30] | J. Cravillon, R. Nayuk, S. Springer, et al., Controlling zeolitic imidazolate framework nano- and microcrystal formation: insight into crystal growth by timeresolved in situ static light scattering, Chem. Mater. 23 (2011) 2130-2141. |
| [31] | S. Hermes, T. Witte, T. Hikov, et al., Trapping metal-organic framework nanocrystals: an in-situ time-resolved light scattering study on the crystal growth of MOF-5 in solution, J. Am. Chem. Soc. 129 (2007) 5324-5325. |
| [32] | S. Diring, S. Furukawa, Y. Takashima, T. Tsuruoka, S. Kitagawa, Controlled multiscale synthesis of porous coordination polymer in nano/micro regimes, Chem. Mater. 22 (2010) 4531-4538. |
| [33] | R. Nayuk, D. Zacher, R. Schweins, et al., Modulated formation of MOF-5 nanoparticles- a SANS analysis, J. Phys. Chem. C 116 (2012) 6127-6135. |
| [34] | D.M. Jiang, T. Mallat, F. Krumeich, A. Baiker, Polymer-assisted synthesis of nanocrystalline copper-based metal-organic framework for amine oxidation, Catal. Commun. 12 (2011) 602-605. |
| [35] | S.K. Nune, P.K. Thallapally, A. Dohnalkova, et al., Synthesis and properties of nano zeolitic imidazolate frameworks, Chem. Commun. 46 (2010) 4878-4880. |
| [36] | A. Imaz, J. Hernando, D. Ruiz-Molina, D. Maspoch, Metal-organic spheres as functional systems for guest encapsulation, Angew. Chem. Int. Ed. 48 (2009) 2325-2329. |
| [37] | G. Lu, S.Z. Li, Z. Guo, et al., Imparting functionality to ametal-organic framework material by controlled nanoparticle encapsulation, Nat. Chem. 4 (2012) 310- 316. |
| [38] | X.M. Lin, G.M. Gao, L.Y. Zheng, Y.W. Chi, G.N. Chen, Encapsulation of strongly fluorescent carbon quantum dots in metal-organic frameworks for enhancing chemical sensing, Anal. Chem. 86 (2014) 1223-1228. |
| [39] | J. An, S.J. Geib, N.L. Rosi, Cation-triggered drug release from a porous zincadeninate metal-organic framework, J. Am. Chem. Soc. 131 (2009) 8376-8377. |
| [40] | J.C. Yu, Y.J. Cui, H. Xu, et al., Confinement of pyridinium hemicyanine dye within an anionic metal-organic framework for two-photon-pumped lasing, Nat. Commun. 4 (2013) 2719. |
| [41] | D. Zacher, O. Shekhah, C. Wö ll, R.A. Fischer, Thin films of metal-organic frameworks, Chem. Soc. Rev. 38 (2009) 1418-1429. |
| [42] | D. Bradshaw, A. Garai, J. Huo, Metal-organic framework growth at functional interfaces: thin films and composites for diverse applications, Chem. Soc. Rev. 41 (2012) 2344-2381. |
| [43] | M. Arnold, P. Kortunov, D.J. Jones, et al., Oriented crystallisation on supports and anisotropic mass transport of the metal-organic framework manganese formate, Eur. J. Inorg. Chem. 2007 (2007) 60-64. |
| [44] | H.L. Guo, G.S. Zhu, I.J. Hewitt, S.L. Qiu, “Twin copper source” growth of metal- organic framework membrane: Cu3(BTC)2 with high permeability and selectivity for recycling H2, J. Am. Chem. Soc. 131 (2009) 1646-1647. |
| [45] | R. Ameloot, L. Stappers, J. Fransaer, et al., Patterned growth of metal-organic framework coatings by electrochemical synthesis, Chem. Mater. 21 (2009) 2580- 2582. |
| [46] | J. Gascon, S. Aguado, F. Kapteijn, Manufacture of dense coatings of Cu3(BTC)2 (HKUST-1) on a-alumina, Microporous Mesoporous Mater. 113 (2008) 132-138. |
| [47] | D.M. Jiang, A.D. Burrows, R. Jaber, K.J. Edler, Facile synthesis of metal-organic framework films via in situ seeding of nanoparticles, Chem. Commun. 48 (2012) 4965-4967. |
| [48] | J.L. Zhuang, D. Ar, X.J. Yu, J.X. Liu, A. Terfort, Patterned deposition of metal-organic frameworks onto plastic, paper, and textile substrates by inkjet printing of a precursor solution, Adv. Mater. 25 (2013) 4631-4635. |
| [49] | W. Cho, H.J. Lee, G. Choi, S. Choi, M. Oh, Dual changes in conformation and optical properties of fluorophores within a metal-organic framework during framework construction and associated sensing event, J. Am. Chem. Soc. 136 (2014) 12201- 12204. |
| [50] | K.K. Yee, N. Reimer, J. Liu, et al., Effective mercury sorption by thiol-laced metal- organic frameworks: in strong acid and the vapor phase, J. Am. Chem. Soc. 135 (2013) 7795-7798. |
| [51] | H.L. Tan, B.X. Liu, Y. Chen, Lanthanide coordination polymer nanoparticles for sensing of mercury(II) by photoinduced electron transfer, ACS Nano 6 (2012) 10505-10511. |
| [52] | Y. Lu, B. Yan, J.L. Liu, Nanoscale metal-organic frameworks as highly sensitive luminescent sensors for Fe2+ in aqueous solution and living cells, Chem. Commun. 50 (2014) 9969-9972. |
| [53] | Y. Zhou, H.H. Chen, B. Yan, An Eu3+ post-functionalized nanosized metal-organic framework for cation exchange-based Fe3+-sensing in an aqueous environment, J. Mater. Chem. A 2 (2014) 13691-13697. |
| [54] | Z.Z. Lu, R. Zhang, Y.Z. Li, Z.J. Guo, H.G. Zheng, Solvatochromic behavior of a nanotubular metal-organic framework for sensing small molecules, J. Am. Chem. Soc. 133 (2011) 4172-4174. |
| [55] | Y. Takashima, V.M. Martínez, S. Furukawa, et al., Molecular decoding using luminescence from an entangled porous framework, Nat. Commun. 2 (2011) 168. |
| [56] | Y.L. Hou, H. Xu, R.R. Cheng, B. Zhao, Controlled lanthanide-organic framework nanospheres as reversible and sensitive luminescent sensors for practical applications, Chem. Commun. 51 (2015) 6769-6772. |
| [57] | W.J. Rieter, K.M.L. Taylor, W.B. Lin, Surface modification and functionalization of nanoscale metal-organic frameworks for controlled release and luminescence sensing, J. Am. Chem. Soc. 129 (2007) 9852-9853. |
| [58] | H. Xu, X.T. Rao, J.K. Gao, et al., A luminescent nanoscale metal-organic framework with controllable morphologies for spore detection, Chem. Commun. 48 (2012) 7377-7379. |
| [59] | H. Xu, F. Liu, Y.J. Cui, B.L. Chen, G.D. Qian, A luminescent nanoscale metal-organic framework for sensing of nitroaromatic explosives, Chem. Commun. 47 (2011) 3153-3155. |
| [60] | C.Y. Zhang, Y.K. Che, Z.X. Zhang, X.M. Yang, L. Zang, Fluorescent nanoscale zinc(II)- carboxylate coordination polymers for explosive sensing, Chem. Commun. 47 (2011) 2336-2338. |
| [61] | R. Li, Y.P. Yuan, L.G. Qiu, W. Zhang, J.F. Zhu, A rational self-sacrificing template route to metal-organic framework nanotubes and reversible vapor-phase detection of nitroaromatic explosives, Small 8 (2012) 225-230. |
| [62] | C.H. Zong, X.J. Liu, H.M. Sun, G. Zhang, L.H. Lu, A new type of nanoscale coordination particles: toward modification-free detection of hydrogen sulfide gas, J. Mater. Chem. A 22 (2012) 18418-18425. |
| [63] | B.X. Liu, Y. Chen, Responsive lanthanide coordination polymer for hydrogen sulfide, Anal. Chem. 85 (2013) 11020-11025. |
| [64] | Z.G. Xie, L.Q. Ma, K.E. deKrafft, A. Jin, W.B. Lin, Porous phosphorescent coordination polymers for oxygen sensing, J. Am. Chem. Soc. 132 (2010) 922-923. |
| [65] | X.L. Qi, S.Y. Liu, R.B. Lin, et al., Phosphorescence doping in a flexible ultramicroporous framework for high and tunable oxygen sensing efficiency, Chem. Commun. 49 (2013) 6864-6866. |
| [66] | S.Y. Liu, X.L. Qi, R.B. Lin, et al., Porous Cu(I) triazolate framework and derived hybrid membrane with exceptionally high sensing efficiency for gaseous oxygen, Adv. Funct. Mater. 24 (2014) 5866-5872. |
| [67] | Y. Lu, B. Yan, A ratiometric fluorescent pH sensor based on nanoscale metal- organic frameworks (MOFs) modified by europium(III) complexes, Chem. Commun. 50 (2014) 13323-13326. |
| [68] | C.B. He, K.D. Lu, W.B. Lin, Nanoscale metal-organic frameworks for real-time intracellular pH sensing in live cells, J. Am. Chem. Soc. 136 (2014) 12253-12256. |
| [69] | C.D.S. Brites, P.P. Lima, N.J.O. Silva, et al., A luminescent molecular thermometer for long-term absolute temperature measurements at the nanoscale, Adv. Mater. 22 (2010) 4499-4504. |
| [70] | Y.J. Cui, H. Xu, Y.F. Yue, et al., A luminescent mixed-lanthanide metal-organic framework thermometer, J. Am. Chem. Soc. 134 (2012) 3979-3982. |
| [71] | A. Cadiau, C.D.S. Brites, P.M.F.J. Costa, et al., Ratiometric nanothermometer based on an emissive Ln3+-organic framework, ACS Nano 7 (2013) 7213-7218. |
| [72] | Z.P. Wang, D. Ananias, A. Carné -Sánchez, et al., Lanthanide-organic framework nanothermometers prepared by spray-drying, Adv. Funct. Mater. 25 (2015) 2824-2830. |
| [73] | C.M. Doherty, G. Grenci, R. Riccò, et al., Combining UV lithography and an imprinting technique for patterning metal-organic frameworks, Adv. Mater. 25 (2013) 4701-4705. |
| [74] | J. Puigmartí-Luis, M. Rubio-Martínez, U. Hartfelder, et al., Coordination polymer nanofibers generated by microfluidic synthesis, J. Am. Chem. Soc. 133 (2011) 4216-4219. |
| [75] | E. Bellido, S. Cardona-Serra, E. Coronado, D. Ruiz-Molina, Assisted-assembly of coordination materials into advanced nanoarchitectures by dip pen nanolithography, Chem. Commun. 47 (2011) 5175-5177. |
 2015, Vol.26
2015, Vol.26 


