b Department of Chemistry, University of Science and Technology of China, Hefei 230026, China
With the development of nanotechnology and related disciplines, the demand for nanostructured materials with designable structures and functions is rapidly growing [1]. Despite many research efforts,to obtain nanomaterials with definable structures and properties by chemical synthesis is highly challenging. DNA nanotechnology as one of the most attractive research frontiers in nanoscience and nanotechnology has a great potential to meet the above need through DNA-guided material assembly [2]. This mainly benefits from the programmable DNA base-pairing coming from the strictest natural selection in living systems. However,to guarantee the accuracy of DNA-programmable assembly,a nanoseparation technique is required for both building block development and product purification.
Gel electrophoresis is a simple and widely adopted tool for biomolecule (e.g. nucleic acids and proteins) isolation. It is heavily relied in molecular biology and genetics laboratories to enable the studies of specific DNA or proteins. In addition to DNA and proteins,the inherent nano-porosity of an agarose gel should allow for nanoseparation applications involving inorganic colloids [3]. In fact,researchers of DNA nanotechnology have been using gel electrophoresis as a simple,cheap,and versatile tool for the separation of DNA-conjugated inorganic nanoparticles and their super-assemblies. This mini review covers some of these innovative applications,including: (1) size and shape separation of colloidal materials; (2) valence separations of DNA-conjugated nanoparticles; and (3) identification and purification of DNAprogrammable nanomaterials.
2. Size and shape sorting of colloidal nanomaterialsOne nice work adopting gel electrophoresis for shape and size discrimination of nanomaterials was demonstrated by Hanauer et al. [4]. As-synthesized silver and gold nanoparticles in mixed shapes (sphere,rod,and triangular plate) were stabilized by polymeric PEG ligands before being loaded on a 0.2% agarose gel. The PEG molecule had a molecular weight of 5000,which was covalently linked with a thiol and a carboxylic group at its two ends. The thiol group provided a strong anchoring of the PEG ligand on the nanoparticles and thus introduced a high density of carboxylic groups. The resulting PEG-capped nanoparticles were well protected from aggregation due to strong electrostatic and steric repulsions. After a voltage was applied on the gel,the silver nanomaterials gradually ran into a broadened band featuring a spectrum of colors (Fig. 1). The different colors stemmed from the shape-dependent plasmonic properties of the metal nanomaterials, allowing a visual observation of the separation process. This meant that nanoparticles with different shapes (and sizes) migrated at slightly different speeds and finally got sorted in the gel. There was also a clear separation between gold spheres (red) and gold rods (green) (Fig. 1). It was important that a suitable terminating group should be chosen for the PEG-loaded nanoparticles in order to realize a successful separation. In this case,- COOH terminated PEG displayed the best separation for the nanoparticles.

|
Download:
|
| Fig. 1.True color agarose gel electrophoretic photograph of Au and Ag nanomaterials with various shapes. Reproduced with permission from Ref. [4]. | |
In the above case,nanoparticles were sorted in an order determined by a mixed size and shape effect (assuming their surface charges were similar). For spherical nanoparticles,it will be much easier to predict their migration order based on their sizes: large particles usually getmore retarded in the gel matrix. Therefore,it is possible to show the size resolution of a gel separation for spherical particles. Another important issue that needs to be addressed is how to scale up the separation for preparative purposes. Xu et al. answered these two questions in their research [5]. They tried a preparative column gel to isolate mixed gold nanoparticles with diameters of 5,15,and 20 nm, and achieved a good separation (Fig. 2a). As shown on a regular slab gel,gold nanoparticles with a diameter difference as small as 2-3 nanometers could be readily resolved (Fig. 2b). In addition,the authors employed the column gel for a shape discrimination of gold nanomaterials. As shown in Fig. 2c,the crude sample was separated into two distinct bands corresponding to spheres and plates (mixed with short rods). Very long rods could not migrate into the gel,which were easily recoverable from the top of the gel. Before all separations,the samples were ligand-exchanged by 11-mercaptoundecanoic acid to produce well-defined gel bands.

|
Download:
|
| Fig. 2.(a) Preparative column agarose gel electrophoretic separation of 5 nm, 15 nm, and 20 nm AuNPs; (b) slab gel electrophoresis of mixed AuNPs of 5 nm, 13 nm, 15 nm, and 18 nm diameters; (c) column gel separation of nanospheres (red), nanoplates (purple), and long nanorods (light brown). Reproduced with permission from Ref. [5]. | |
DNA-conjugated nanoparticles are important building blocks for DNA-programmable nanofabrication. One fundamental prerequisite is that the nanoparticles should have a strictly defined DNA valence (number of DNA ligands per particle) in order to realize an accurate assembly control. Unfortunately,these ideal building blocks are hardly achievable in a DNA-conjugation reaction. Instead,a wide distribution of DNA valences are normally obtained for the DNA-nanoparticle conjugates. The introduction of a nanoseparation technique may help to solve this problem.
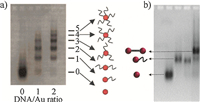
The isolation of Au nanoparticles (AuNPs) decorated with a specific number of DNA oligonucleotides was shown by Zanchet et al. in 2001 [6]. Because the gel mobility of a DNA-nanoparticle conjugate is dictated by its size and surface charge,it is important that unconjugated AuNPs should have a stable and negative surface charge close to or higher than DNA to realize a DNA valence separation. This condition can be met after modifying the AuNPs with a suitable capping agent. In this way,the introduction of a DNA strand on an AuNP will cause a decreased gel mobility due to an increase of its hydrodynamic size. As a result,DNA-AuNP conjugates with mixed valences could be separated into a ladder of gel bands (Fig. 3a). It should be emphasized that monovalent DNA- AuNP products are of special interest for DNA-templated assembly, though all other valences can have different uses. For example, AuNP dimers could be assembled in very high yields by hybridizing two DNA-AuNP mono-conjugates (Fig. 3b) [7]. Following this pioneering work,complicated AuNP superstructures have been made based on DNA programming [2, 8].
|
Download:
|
| Fig. 3.(a) Electrophoretic valence separation of 5 nmDNA–AuNP conjugates; (b) gel purification of AuNP dimers. Reproduced with permissions from Refs. [6,7]. | |
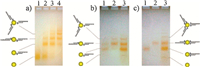
It is now known that nanoparticles should have a narrow size distribution,a good stability,and a high surface charge in order to realize a gel-based separation. These restrictions have caused an obvious delay in adapting the agarose gel electrophoretic separation to other metal materials including Ag nanoparticles (AgNPs). Despite the unique chemical,electronic,and optical properties of AgNPs,the weak chemical and colloidal stabilities coupled with the synthetic difficulties of monodisperse AgNPs have made their DNA conjugation quite tricky [9, 10, 11]. Zheng et al. [12] synthesized 2 nmAgNPs with excellent stability by employing shortened fish sperm DNA (FSDNA) as a nucleation template. Thanks to the existence of the surface-adsorbed FSDNA,a very sharp gel band was observed for the AgNPs. More importantly,the as-obtained AgNPs could be easily decorated with thiolated DNA, based on which a clear DNA valence separation was realized (Fig. 4a). Besides,the gel results clearly revealed that a single thiol group was not enough to guarantee a strong bonding between DNA and AgNPs. Accordingly,a re-distribution of the purified DNA valences was observed from a purity check gel (Fig. 4b). This problem was solvable by attaching two thiols on the same terminus of the double-stranded DNA ligand (Fig. 4c).
|
Download:
|
| Fig. 4.(a) Gel electrophoresis of DNA conjugated AgNPs (lanes 2–4 corresponding to increased DNA:nanoparticle ratio); (b and c) purity check of isolated monovalent (lane 1) and divalent (lane 2) DNA–AgNPs. One and two thiols were appended to the DNA ligands in (b) and (c), respectively. Reproduced with permission from Ref. [12]. | |
While gold and silver nanoparticles are famous due to their size-dependent optical properties,Pt nanoparticles (PtNPs) are very important catalysts. Synthesis of PtNPs with specified DNA valences will provide important building blocks for DNA-programmable catalytic materials. Li et al. [13] found that bis (psulfonatophenyl) phenylphosphine dipotassium salt (BSPP) was an effective capping agent to increase the stability and surface charge of citrate-protected PtNPs (Fig. 5a). The BSPP-stabilized PtNPs with a diameter of 3-4 nm bearing specific numbers (up to 7) of DNA strands could be clearly separated on an agarose gel (Fig. 5b). The resulting valence-pure DNA-PtNPs enabled the assembly of molecule-like "heteroatom" nanoparticle clusters with formulas of Au1Pt1,Au2Pt1,Au3Pt1,Au1Pt2,and Au1Pt3.

|
Download:
|
| Fig. 5.(a) Agarose gel electrophoresis of unconjugated PtNPs and AuNPs capped by different agents; (b) valence separation of DNA–PtNP conjugates. Reproduced with permission from Ref. [13]. | |
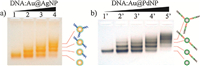
The successes with Au,Ag,and Pt nanoparticles have encouraged the generalization of gel separation to more inorganic materials. Wang et al. developed an AuNP-seeded core-shell strategy for DNA functionalization of non-gold metal materials with a great potential to achieve this goal [14]. This method has several advantages: (1) it is much easier to synthesize monodisperse core-shell structures with different shell compositions; (2) the facile synthesis and surface decoration of AuNPs make it easy to control the colloidal properties of as-synthesized core-shell products; (3) the interaction between the gold core and a nongold shell provides extra chances to fine-tune their physicochemical properties. Au@Ag and Au@Pd core-shell structures were taken to demonstrate this idea [14]. In the case of Au@AgNPs,dT10 DNAcoated AuNPs were used as the nucleation seeds,which provided a highly charged surface and a good stability of the Au@Ag structures. The as-synthesized Au@AgNPs appeared as a sharp band during gel electrophoresis (Fig. 6a,lane 1). Further conjugation of the Au@AgNPs with long DNA sequences led to a ladder of separated gel bands representing different DNA valences (Fig. 6a,lanes 2-4). In contrast to the Au@AgNPs,Au@PdNPs required a BSPP coating to obtain a well-defined band in the gel (Fig. 6b,lane 10). Note that BSPP is not a suitable capping agent for AgNPs due to its silver etching behavior in the presence of dissolved O2. The as-synthesized Au@PdNPs (despite the irregular shape of the Pd layer) were suitable for a high resolution agarose gel separation of different DNA valences (Fig. 6b,lanes 20-50). The Au@Ag and Au@Pd core-shell nanoparticles with purified DNA valences are useful building blocks for DNA-programmable nongold metamaterials.
|
Download:
|
| Fig. 6.Agarose gel electrophoretic DNA valence separations of DNA-conjugated Au@AgNPs (a) and Au@PdNPs (b). Reproduced with permission from Ref. [14]. | |
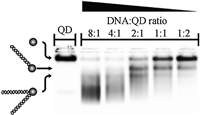
Besides metal nanoparticles,DNA-conjugated quantum dots (QDs) can been similarly isolated by agarose gel electrophoresis. In a work by Carstairs et al. streptavidin coated QDs were conjugated by single or double stranded DNA (end-tagged with a biotin moiety) [15]. As shown in Fig. 7,QDs bearing increased DNA valences migrated at a faster speed,in opposite to the case of Au, Ag,or Pt nanoparticles discussed above. It was possible (since the polarity of electrophoresis was not indicated in this paper,we assume a normal upward electric field) that an introduction of DNA increased the surface charge of the QDs,and led to increased gel mobility. Similar phenomenon was observed in an earlier publication [16].
|
Download:
|
| Fig. 7.Gel electrophoretic valence separation of DNA-QD conjugates. Note that DNA increased the migration speed of streptavidin-coated QDs. Reproduced with permission from Ref. [15]. | |
The above work clearly shows that gel electrophoresis is a useful technique for the separation of individual nanoparticles according to their size,shape,charge,and DNA valence. In addition to these applications,other versatile uses of agarose gel electrophoresis have been demonstrated in DNA nanotechnology. For example,gel electrophoresis has offered an efficient tool to identify and isolate DNA-assembled nanomaterials.
Despite the high accuracy of DNA-guided assembly,incomplete as well as erroneous products still exist,which have to be removed from the products. In the case of nanoparticle clusters with a finite size,it is possible to precisely identify a desired product with single nanoparticle accuracy based on a gel separation. Zanchet et al. showed that dimeric and trimetric nanoparticle clusters could be easily resolved during a gel separation and got purified [7]. Fu et al. relied on agarose gel electrophoresis to isolate various possible assemblies between AuNPs and QDs [16]. Deng et al. successfully purified a series of AuNP oligomers assembled on a linear DNA template [17]. A recent work by Wang et al. clearly demonstrated the resolving power of agarose gel for linear AuNP assemblies containing up to 6 AuNPs [18]. Based on this work [18],it was interesting that hexamers could be assembled as a dominant product for 5 nm AuNPs,while a series of incomplete assemblies formed for 13 nm AuNPs. This could be attributed to a "crowding" effect during the assembly of large AuNPs.
In the case of an infinite nanoparticle assembly,a single nanoparticle missing could not be resolved. As a result,a gelisolated product may not be a 100% equivalent to an expected structure. Therefore,excess amount of nanoparticle building blocks should be added to achieve a saturated assembly. For instance,a rolling-circle amplified [19] single stranded DNA was employed to template the assembly of micrometer long AuNP linear arrays [17]. In this case,gel electrophoresis could not resolve the exact composition of the polydisperse product which actually contained different numbers of nanoparticles. Besides a linear array,gel electrophoresis was employed to prepare monodisperse core-satellite structures [20]. Satellite AuNPs with minimum DNA decoration (not monovalent) were added in large excess to heavily DNA-decorated (with complementary sequence) core particles so that crosslinked multi-core products could be largely suppressed [20]. DNA origami [21, 22, 23] has also been employed to guide nanoparticle assembly. Because of the large molecular weight of a DNA origami,it is often hard to resolve a single nanoparticle difference on its surface. Therefore,excessive nanoparticles were used to achieve saturated assembly,with the product being purified by gel electrophoresis [24, 25, 26, 27, 28].
Apart from nanoparticles,Li et al. [29] developed a novel type of DNA-grafted single walled carbon nanotubes (SWNTs) with high hybridization activity by controlling nonspecific DNA binding on SWNTs [30]. Gel electrophoresis was then employed to monitor the hybridization of the DNA-conjugated SWNTs [29].
5. Summary and outlookWe have summarized some typical applications of agarose gel electrophoresis in DNA nanotechnology,including size,shape,and DNA-valence separations of individual nanoparticles as well as the identification and purification of their assemblies. While these applications will definitely continue to help,a deep insight into agarose gel electrophoretic nanomaterial separation is obviously needed. Compared with regular DNA and protein samples, nanomaterials are much more complicated from the separation point of view. The wide distributions of composition,shape,size, charge,and stability of chemically synthesized nanomaterials require careful theoretical and experimental considerations for a successful separation. Previous work has told us that many factors including synthetic conditions,surface capping agent,DNA anchoring group,and separation parameters may dramatically affect a separation. However,no simple rules can be followed in order to achieve the best results. Accordingly,compromises among size,shape,charge,stability,and other properties of nanomaterials are often made in order to achieve a satisfactory separation. Such a situation has to be improved in the future to broaden the applications of agarose gel electrophoresis as a nanoseparation tool in many related fields including DNA nanotechnology.
AcknowledgmentsThis work was supported by NNSFC (Nos. 21273214,21425521, 21521001),Hefei Center for Physical Science and Technology (No. 2014FXCX010),and Collaborative Innovation Center of Suzhou Nano Science and Technology.
| [1] | Z.H. Nie, A. Petukhova, E. Kumacheva, Properties and emerging applications of self-assembled structures made from inorganic nanoparticles, Nat. Nanotech. 5 (2010) 15-25. |
| [2] | M.R. Jones, N.C. Seeman, C.A. Mirkin, Programmable materials and the nature of the DNA bond, Science 347 (2015) 1260901. |
| [3] | N. Surugau, P.L. Urban, Electrophoretic methods for separation of nanoparticles, J. Sep. Sci. 32 (2009) 1889-1906. |
| [4] | M. Hanauer, S. Pierrat, I. Zins, et al., Separation of nanoparticles by gel electrophoresis according to size-and shape, Nano Lett. 7 (2007) 2881-2885. |
| [5] | X.Y. Xu, K.K. Caswell, E. Tucker, et al., Size and shape separation of gold nanoparticles with preparative gel electrophoresis, J. Chromatogr. A 1167 (2007) 35- 41. |
| [6] | D. Zanchet, C.M. Micheel, W.J. Parak, et al., Electrophoretic isolation of discrete Au nanocrystal/DNA conjugates, Nano Lett. 1 (2001) 32-35. |
| [7] | D. Zanchet, C.M. Micheel, W.J. Parak, et al., Electrophoretic and structural studies of DNA-directed Au nanoparticle groupings, J. Phys. Chem. B 106 (2002) 11758- 11763. |
| [8] | S.J. Tan, M.J. Campolongo, D. Luo, W.L. Cheng, Building plasmonic nanostructures with DNA, Nat. Nanotechnol. 6 (2011) 268-276. |
| [9] | J.S. Lee, A.K.R. Lytton-Jean, S.J. Hurst, C.A. Mirkin, Silver nanoparticle-oligonucleotide conjugates based on DNA with triple cyclic disulfide moieties, Nano Lett. 7 (2007) 2112-2115. |
| [10] | S. Pal, J. Sharma, H. Yan, Y. Liu, Stable silver nanoparticle-DNA conjugates for directed self-assembly of core-satellite silver-gold nanoclusters, Chem. Commun. (2009) 6059-6061. |
| [11] | C. Lin, H. Gong, L.Z. Fan, X.H. Li, Application of DNA/Ag nanocluster fluorescent probe for the detection of Pb2+, Acta Chim. Sin. 72 (2014) 704-708. |
| [12] | Y.Q. Zheng, Y.L. Li, Z.X. Deng, Silver nanoparticle-DNA bionanoconjugates bearing a discrete number of DNA ligands, Chem. Commun. 48 (2012) 6160-6162. |
| [13] | Y.L. Li, Y.Q. Zheng, M. Gong, Z.X. Deng, Pt nanoparticles decorated with a discrete number of DNA molecules for programmable assembly of Au-Pt bimetallic superstructures, Chem. Commun. 48 (2012) 3727-3729. |
| [14] | H.Q. Wang, Y.L. Li, M. Gong, Z.X. Deng, Core solution: a strategy towards gold core/ non-gold shell nanoparticles bearing strict DNA-valences for programmable nanoassembly, Chem. Sci. 5 (2014) 1015-1020. |
| [15] | H.M.J. Carstairs, K. Lymperopoulos, A.N. Kapanidis, J. Bath, A.J. Turberfield, DNA monofunctionalization of quantum dots, ChemBioChem 10 (2009) 1781-1783. |
| [16] | A. Fu, C.M. Micheel, J. Cha, et al., Discrete nanostructures of quantum dots/Au with DNA, J. Am. Chem. Soc. 126 (2004) 10832-10833. |
| [17] | Z.X. Deng, Y. Tian, S.H. Lee, et al., DNA-encoded self-assembly of gold nanoparticles into one-dimensional arrays, Angew. Chem. Int. Ed. 44 (2005) 3582-3585. |
| [18] | H.Q. Wang, Y.L. Li, M. Liu, M. Gong, Z.X. Deng, Overcoming the coupling dilemma in DNA-programmable nanoparticle assembly by “Ag + soldering”, Small 11 (2015) 2247-2251. |
| [19] | P. Liu, X.H. Yang, Q. Wang, et al., Sensitive detection of DNA methyltransferase activity based on rolling circle amplification technology, Chin. Chem. Lett. 25 (2014) 1047-1051. |
| [20] | Y. Yang, X. Bai, L.L. Fang, Z.X. Deng, Fabrication of monodisperse ‘core-satellite' nanostructures by DNA-programming: a novel class of superstructured building blocks for hierarchical nanoassembly, Chin. J. Chem. Phys. 26 (2013) 601-606. |
| [21] | P.W.K. Rothemund, Folding DNA to create nanoscale shapes and patterns, Nature 440 (2006) 297-302. |
| [22] | T. Liedl, B. Hö gberg, J. Tytell, et al., Self-assembly of three-dimensional prestressed tensegrity structures from DNA, Nat. Nanotechnol. 5 (2010) 520-524. |
| [23] | D.R. Han, S. Pal, J. Nangreave, et al., DNA origami with complex curvatures in three-dimensional space, Science 332 (2011) 342-346. |
| [24] | Z. Zhao, E.L. Jacovetty, Y. Liu, H. Yan, Encapsulation of gold nanoparticles in a DNA origami cage, Angew. Chem. Int. Ed. 50 (2011) 2041-2044. |
| [25] | X.B. Shen, C. Song, J.Y. Wang, et al., Rolling up gold nanoparticle-dressed DNA origami into three-dimensional plasmonic chiral nanostructures, J. Am. Chem. Soc. 134 (2012) 146-149. |
| [26] | X.B. Shen, A. Asenjo-Garcia, Q. Liu, et al., Three-dimensional plasmonic chiral tetramers assembled by DNA origami, Nano Lett. 13 (2013) 2128-2133. |
| [27] | A. Kuzyk, R. Schreiber, Z. Fan, et al., DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response, Nature 483 (2012) 311-314. |
| [28] | X. Lan, Z. Chen, G.L. Dai, et al., Bifacial DNA origami-directed discrete, threedimensional, anisotropic plasmonic nanoarchitectures with tailored optical chirality, J. Am. Chem. Soc. 135 (2013) 11441-11444. |
| [29] | Y.L. Li, X.G. Han, Z.X. Deng, Grafting SWNTs with highly hybridizable DNA sequences: potential building blocks for DNA-programmed material assembly, Angew. Chem. Int. Ed. 46 (2007) 7481-7484. |
| [30] | Y. Wang, D.M. Zhou, Z. Wu, et al., Terminal protection of small molecule-linked ssDNA-SWNT nanoassembly for sensitive detection of small molecule and protein interaction, Chin. Chem. Lett. 24 (2013) 107-110. |
 2015, Vol.26
2015, Vol.26 


