In recent years,nanoparticle synthesis and applications attracted the attention of scientists from all parts of the world due to the catalytic,thermal,optical and electrical properties of the nano-sized colloids. Nanoparticles can be used in various applications such as conductors,chemical sensors,catalysts, conducting ink,biosensor,antibacterial activity,etc. [1, 2, 3, 4, 5, 6, 7, 8]. With the increasing resistance of microbial organisms to multiple antibiotics and the pressure on the health care costs,much research was initiated to overcome such problems; which led to a resurgence in the use of silver-based antibiotics to overcome the microbial resistance to antibiotics. Silver nanoparticles (AgNPs) are used to control bacterial growth in a many applications,like dental work,catheters,and burn wounds [9]. Silver ions and Ag-based compounds are highly toxic to microorganisms,showing strong biocidal effects on bacteria [10]. Recently,for instance,Mecking and co-workers showed that hybrids of Ag nanoparticles with amphiphilic,hyper-branched macromolecules exhibited effective antimicrobial surface coating agents [11].
Several methods were developed for nano-colloidal preparations, some of them are biological [12],electrochemical [13], photochemistry [14, 15, 16],microemulsion [17] and microwave techniques [18]. In wet chemical synthesis,using an aqueous system provides the condition for large scale and lowest costeffective synthesis of metal nanoparticles. Some reducing agents, such as citrate,sodium borohydrate,ammonia,and hydrogen, were used in an aqueous system for AgNP synthesis using surfactants for controlling the growth of the formed nanoparticles [19].
Our work focused on developing a simple and an effective green approach toward the synthesis and stabilization of AgNPs. Sunlight is used as the reducing agent with prepared cationic surfactants. The utilized surfactants act as a stabilizing agent for the synthesized AgNPs and assist in the reduction process. The prepared silver nanoparticles were confirmed using transmission electron microscopy,dynamic light scattering,and UV-vis spectroscopy. The prepared silver nanoparticle encapsulated with the cationic surfactant (antibiotic),were tested against Gram positive and Gram-negative bacteria and also sulfate reducing bacteria.
2. Experimental 2.1. ChemicalsSilver nitrate (AgNo3,99%) used in the preparation of the silver nanoparticles was provided by Sigma-Aldrich/Germany. The chemicals used in the preparation of capping agents were dimethylaminopropylamie (DMAPA),butyraldeyde,decyl bromide, dodecyl bromide and hexadecyl bromide and were purchased from Aldrich Chemicals Co.,Ltd.
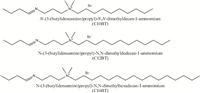
2.2. Synthesis 2.2.1. Preparation of cationic capping agentsThe utilized cationic capping agents were previously reported [20]. The chemical structures of prepared capping agents are shown in Scheme 1.
|
Download:
|
| Scheme. 1.The chemical structure of prepared cationic capping agents. | |
Sunlight was used in the preparation of silver nanoparticles from an aqueous solution of silver nitrate. The reducing agent was sunlight with the assistance of the prepared cationic surfactants [14]. A 20 mL aliquot of a 2 mmol/L aqueous solution of AgNO3was mixed with 20 mL of a 2 mmol/L cationic surfactant aqueous solution,then the mixed solution was exposed to direct sunlight (Scheme 2). After a short time,over 5 min at maximum,the color of the solution changed from colorless to yellow within different ranges,depending on the used capping agents as shown in Fig. 1.
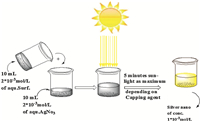
|
Download:
|
| Scheme. 2.In situ photo preparation of silver nanoparticles. | |
|
Download:
|
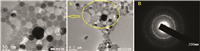
| Fig. 1.(A) TEM image of prepared silver nanoparticle capped by (C10BT), (B) is SAED image of prepared silver nanoparticles capped by (C10BT). | |
The following instrumentations have been used for silver nanoparticle confirmation:
Transmission electron microscope (TEM): A convenient way to produce good TEM samples is to use copper grids,specifically a copper grid pre-covered with a very thin amorphous carbon film. To investigate the prepared AgNPs using TEM,small droplets of the liquid were placed on the carbon-coated grid [21]. A photographic plate of the transmission electron microscopy is employed in the present work to investigate the microstructure of the prepared samples using TEM model "JeolJeM-2100 (Japan)" (Egyptian Petroleum Research Institute "EPRI").
UV-visible spectroscopy: The formation of silver nanoparticles was confirmed using UV-visible spectrophotometer (Shimadzu, UV-2550,Japan) [22].
Dynamic light scattering (DLS): The hydrodynamic diameter and zeta potential of the prepared AgNPs capped with the prepared cationic surfactant were characterized by dynamic light scattering (DLS) using a Malvern Zetasizer Nano (Malvern Instruments Ltd, Worcestershire,UK). Each DLS measurement was run in triplicate using automated,optimal measurement time and laser attenuation settings. The recorded correlation functions and measured mobilities of particles were converted into size distributions and zeta potentials,respectively,using the Malvern Dispersion Software (V5.10,http://www.zetasizer.com/).
Fourier transform infrared spectrometer (FTIR): FT-IR spectra were recorded using the obtained solid cationic surfactants capped AgNPs after centrifugation and washing to remove the unassociated organic molecules [23]. Spectra were recorded on an ATI Mattson Infinity SeriesTM,Bench Top 961 instrument controlled by Win FirstTM V2.01 software (Egyptian Petroleum Research Institute "EPRI").
2.4. Biological activityBiological activity against a wide range of bacteria and fungi: Different species of tested organisms were obtained from the Operation Development Center,Egyptian Petroleum Research Institute. The bacteria species were grown on nutrient agar,while fungi mold on Czapek’s Dox agar. Nutrient agar consists of beef extract (3.0 g/L),peptone (5.0 g/L),sodium chloride (3.0 g/L) andagar (20.0 g/L),then,diluted the volume to one liter,heated the mixture to the boiling,and sterilize the media by autoclave. Czapek’s Dox agar consists of sucrose (20.0 g/L),sodium nitrate (2.0 g/L),magnesium sulfate (0.5 g/L),potassium chloride (0.5 g/L), ferrous sulfate (0.01 g/L) and agar (20.0 g/L),then,diluted the volume to 1 L,heated the mixture to the boiling,and sterilize the media by autoclave.
The tested microorganisms were Gram-positive bacteria (Bacillus pumilus and Micrococcus luteus),Gram-negative bacteria (Pseudomonas aeuroginosa and Sarcina lutea) and Fungi (Candida albicans and Penicillium chrysogenum).
The filter-paper disk-agar diffusion (Kirby-Bauer) method was used to determine the ability of an antibiotic to kill,or inhibit,the growth of living microorganisms [24].
The procedures of the testwere as follows: (1) Inoculate a flask of melted agarmediumwiththe organismtobe tested,andthenpour it in a covered petri dish; (2) the three different antibioticswere laidon top of agar. The inhibition zone diameterswere measured after 48 h at 36 ± 1 °C (for bacteria) and four days at 36 ± 1 °C (for yeast and fungi); (3) biocidal activity against sulfate reducing bacteria: The inhibition activity of the prepared silver in the nano-form against the sulfate reducing bacteria (SRB) growth was measured using the serial dilutionmethod according to ASTMD4412-84 [25]. SRB-contaminated water was supplied from Qarun Petroleum Co. (West Desert,Egypt). This water was used for microbial inhibition test. The test has been subject to growth of about 1015 bacteria cell/mL. The prepared compounds were tested as a biocide for the SRB by doses of (5 × 10-4,1 × 10-4,7 × 10-5 and 5 × 10-5 mol/L). The system was incubated for a contact time of 3.0 h; each systemwas cultured in SRB specific media for 21 days at 37-40 °C.
3. Results and discussionsAqueous solution of AgNO3 was reduced using sunlight as the reducing agent,as a gratis source. The prepared cationic surfactant used as capping agent and accelerating agent in the reduction process. The steps of the reaction are shown in Scheme 2. In a control experiment,when the sample was stored in dark to exclude light,the solution did not change color,or form any solid precipitate over a longer period. When the silver nitrate solution exposed to sunlight without capping agent,after long time exceeding two months,we observed a very slight change in color with very small precipitates on walls of the glass vial; an indication of nanoparticle formation.
The general mechanism for the synthesis of AgNPs,comprise of a two-step process,i.e.,atom formation and then polymerization of the formed atoms. The presence of prepared cationic surfactant in the reaction prevent further coalescence and aggregation [26, 27]. When aqueous solutions subject to sunlight irradiation (g-radiolysis),it produces the following species [14]:
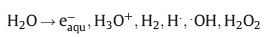
The solvated electrons and H• free radical atoms are strong reducing agents,reducing the silver ions to silver atoms in the nano-form:
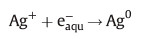
So both oxidizing OH• radicals and H produced in the radiolysis of water should be scavenged,which can be done efficiently by capping agents to produce H2O,H2 and an organic surfactant radical [28, 15].
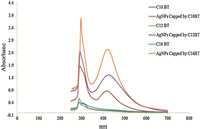
3.1. Confirmation of silver nanoparticle formationStable silver nanoparticles were preparedwiththe shape and size distribution characterized using TEM,SAED,UV,DLS and FTIR techniques. The morphology of AgNPs was investigated by transmission electron microscopy (Fig. 1A). TEM photographs indicate that the nanosilver solution consists of well dispersed agglomerates of spherically shaped nanoparticles. Fig. 1B shows the selected area electron diffraction pattern of capped AgNPs (SAED) by C10BT surfactants,indicating that the prepared AgNPs are polycrystalline [29, 30, 31]. UV-vis spectroscopy is quite sensitive to the formation of silver nanoparticles due to surface plasmon excitation [32, 33]. Fig. 2 shows the absorption spectra of AgNPs capped by prepared surfactants,having absorption bands at λmax 416,424 and 416 nm for AgNPs capped by C10BT,C12BT and C16BT surfactants,respectively,indicating formation of AgNPs due to surface plasmon resonance of colloidal silver nanoparticles [34]. Bands at λmax 288,290 and 292 nm,characteristic of the used capping agents C10BT,C12BT and C16BT,respectively,matches with the bands appearing in an aqueous solution of the capping agents alone. It is known that the amount and size of AgNPs are positively related to the adsorption peak intensity and the λmax on the UV-vis spectra [35, 36],respectively. Data presented in Fig. 2, show an increase in the absorbance of bands of prepared AgNPs with increasing hydrophobic chain length of the used capping agent which provides evidence for increasing the amount of the silver nanoparticles formed by increasing the later. For example, the absorbance of AgNPs capped by C10BT,C12BT and C16BT were 0.87,1.51 and 2.53,respectively. Changing the chain length of the capping agent has a slight effect on the shift λmax from which we can conclude that prepared AgNPs by this method give similar size distribution which is confirmed by dynamic light scattering data in Table 1.
|
Download:
|
| Fig. 2.UV spectra of prepared silver nanoparticles using C10BT, C12BT and C16BT as capping agent. | |
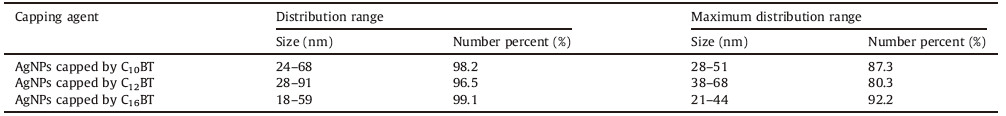
| Table 1 Size distribution of prepared silver nanoparticles using prepared capping agents. |
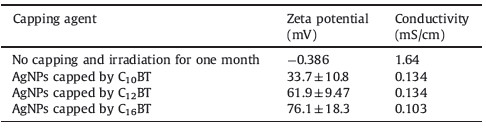
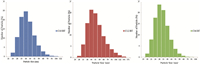
Particle size distribution of prepared in aqueous solution using different capping agents is depicted in Table 1,and shown in Fig. 3. The method of AgNPs preparation produces a similar size distribution and this result matches data obtained from UV-vis spectroscopy as shown in Fig. 2 [23, 37, 22]. By inspection of data in Table 2,it was found that the zeta potential values of capped AgNP susingthe prepared cationic surfactants are greater than +30 mV, which is an indication of the high stability of prepared AgNPs against agglomeration [38]. The high value of the zeta potential also indicates the electrostatic repulsion between the formed particles increase,keeping particles free from agglomeration and stable for long periods. The acquired positive charge of zeta potential is mainly due to the used capping agent as a cationic surfactant (carry positive charge) [39],while the zeta potential of silver nanoparticles prepared without using a capping agent after irradiation to sunlight for one month was -0.386 mV. Increasing the hydrophobic chain length of the capping agent increases the stability of the formed nanoparticles as indicated by the values of zeta potential in Table 2. For example,the zeta potentials of prepared silver nanoparticles capped by C10BT,C12BT and C16BT are 33.7 ± 10.8,61.9 ± 9.47 and 76.1 ± 18.3,respectively.
|
Download:
|
| Fig. 3.Size distribution of silver nanoparticles using prepared capping agents determined by DLS. | |
| Table 2 Zeta Potential and conductivity of prepared silver nanoparticle by dynamic light Scattering (DLS). |
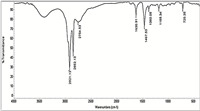
FT-IR spectroscopy is used in order to understand the role of prepared capping agents in the formation of silver nanoparticles and the chemical environment of the final product. In Fig. 4,all bands around 2926,2851 and 1626 cm-1 indicate the presence of a capping agent with the nanoparticles. Bands at 2928 and 2851 cm-1 correspond to an asymmetric and symmetric C-H stretches of the alkyl chain of C16BT. The band at 1626 cm-1 corresponds to the Schiff base group (-C55N-). On comparing IR bands of AgNPs capped by surfactants in Fig. 4 with IR bands of surfactant alone in Fig. 5,we note that some band maxima are slightly blue shifted and some bands are also slightly red shifted. These relatively small shifts are mostly due to the constraint of the capping molecular motion,which presumably resulted from the attachment on the nanoparticle surfaces [40, 41, 42, 43]. Also,we note an increase in the intensity of the band around 1377 cm-1 by 50%-80% than a band of the surfactant alone,which indicates the presence of a co-ordination bond between -CH2- of the hydrophobic chain length of the capping agent and the surface of the silver nanoparticles. This co-ordination bond enhances the role of the chain length of the capping agents in shaping, sizing and distribution,as indicated by both transmission electron microscopy,UV-vis spectroscopy and dynamic light scattering.

|
Download:
|
| Fig. 4.FTIR spectrum C16BT capped silver nanoparticles. | |
|
Download:
|
| Fig. 5.IR spectrum of N-(3-(butylideneamino)propyl)-N,N-dimethylhexadecan-1- ammonium (C16BT). | |
The potency of prepared silver nanoparticles capped by cationic surfactants as antibacterial and antifungal agents against some pathogenic Gram-positive (Bacillus pumilus and Micrococcus luteus) and Gram-negative (Pseudomonas aeuroginosa and Sarcina lutea) bacteria and some pathogenic fungi (Candida albicans and Penicillium chrysogenum) were tested and compared with surfactants alone. The tested bacteria have some effect on the human health,like Bacillus subtilis (causes disease in severely immune compromised patients,and can conversely be used as a probioticin healthy individuals),Micrococcus (rarely causes infections or problems in the body,those with compromised immune systems), Pseudomonas aeruginosa (can cause infection of the blood (bacteremia),heart (endocraditis),central nervous system (meningitis, brain abscess),ear (otitis externa,or swimmer’s ear),eyes, bones,joints,skin,urinary,and gastrointestinal tract) and Candida albicans (the organism responsible for most fungal infections and can cause symptoms when a weakened immune system).
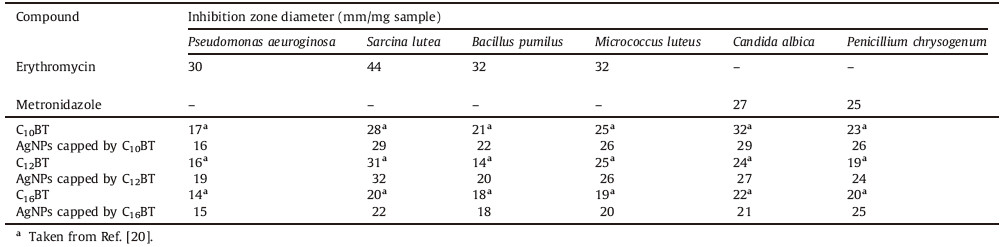
The results of antimicrobial activity are recorded in Table 3, indicating that the synthesized compounds are very good antibiotics compared to the references. Even some cationic surfactants and their silver nanoparticles possess biological higher than the used reference,especially against fungi. From the data in Table 3,it was found that the activity of C10BT capped AgNPs was higher than the two other series i.e.,the biological activity is a nonlinear dependence on the chain of the surfactants [44, 45]. These results agree with the results obtained previously [46, 47],with this phenomenon described as the cut-off effect [48]. Several theories discussed this effect based on some factors like the critical micelle concentration of surfactants,the change in free energy of adsorption of the surfactant,solubility and size. The critical micelle concentration of C10BT is the higher [20],therefore,the concentration at the cell membrane is lower,thus lower activity is predicted. From the point view of thermodynamics,the tendency of C16BT to adsorb at a cell membrane is higher,so it is predicted to have higher efficiency [20]. Other accounts attribute this cut-off to a decrease in perturbation of the membrane at higher chain lengths,proposing that the longer alkyl chain molecules better mimic molecules in the lipid bilayer,causing less of a disruption in the membrane [49]. By increasing the alkyl chain of surfactants, lipid solubility increases at a rate faster than the change in partition coefficient (lipid/aqueous) theory; hence C10BT has higher activity [50]. Finally we can attribute the cut-off effect to the magnitude of all previous parameters which appear in Table 3, in which the C10BT capped AgNP is more effective as an antibacterial and antifungal agent. By comparing the biological activity of the tested surfactant capped AgNPs in Table 3 with data on the surfactant,as in data from Ref. [20],we note that the antibacterial and antifungal activity of the nano-form is higher,for example,the activity of C10BT capped AgNPs against Penicillium chrysogenum fungi is 26 mm/mg,while C10BT is 23 mm/mg [20]. This can be attributed to silver nanoparticle alone for the biological activity,so prepared surfactant capped AgNPs have higher activity which can be attributed to the higher surface area of prepared nanoparticles and the acquired positive charge of prepared silver nanoparticles (as indicated in zeta potential values in Table 2,in addition to the positive charge of cationic surfactants where these positive charges facilitate adsorption at the negative cell wall membrane of bacteria. Sulfate Reducing Bacteria (SRB) produces H2S and causes a large problem in the petroleum sector by increasing the corrosiveness of brine and causing metals to crack and blister. The prepared cationic surfactant capped AgNPs show very good effectiveness against SRB,as indicated in Table 4. SRB-contaminated water used for the microbial inhibition test has been subject to growth of about 1015 bacteria cell/mL. By inspection of data in Table 4,we can observe that prepared cationic surfactant capped silver nanoparticles have higher activity compared to using a cationic surfactant,as it is,where we can obtain on the same activity at a concentration of surfactant cappedsilver nanoparticles lower by fivefold than the surfactant alone. For example,C16BT capped silver nanoparticles gives SRB account equal Nil cell/mL at concentration 1 × 10-4 mol/L,while we can obtain the same activity for C16BT alone,but at concentration 5 × 10-4 mol/L [20].
| Table 3 Antimicrobial activity of synthesized surfactants and their nano form against pathogenic bacteria and fungi. |
| Table 4 Biocidel effect of the prepared compounds against sulfate reducing bacteria, SRB. |
Silver nanoparticles were prepared by a green and rapid method using surplus materials. The prepared cationic surfactant showed great effect through the process of nanoformation through contribution to the reduction method and through their role as capping agent,which is confirmed by DLS,UV-vis and FTIR spectroscopy. By increasing the hydrophobic chain length of the capping agents,the stability of capped AgNPs increase as indicated by increasing zeta potential values. Increasing the hydrophobic chain length of the capping agents,the amount of AgNPs increase (Increasing absorbance in UV-vis spectra). The silver nanoparticles increase the biological activity of the capping agents.
| [1] | A.J. Haes, R.P. van Duyne, Nanosensors enable portable detectors for environmental and medical applications, Laser Focus World 39 (2003) 153-156. |
| [2] | S. Magdassi, A. Bassa, Y. Vinetsky, A. Kamyshny, Silver nanoparticles as pigments for water-based ink-jet inks, Chem. Mater. 15 (2003) 2208-2217. |
| [3] | F. Frederix, J.M. Friedt, K.H. Choi, et al., Biosensing based on light absorption of nanoscaled gold and silver particles, Anal. Chem. 75 (2003) 6894-6900. |
| [4] | A.A. Abd-Elaal, S.M. Tawfik, S.M. Shaban, Simple one step synthesis of nonionic dithiol surfactants and their self-assembling with silver nanoparticles: characterization, surface properties, biological activity, Appl. Surf. Sci. 342 (2015) 144- 153. |
| [5] | S. Gamerith, A. Klug, H. Scheiber, et al., Direct ink-jet printing of Ag-Cu nanoparticles and Ag-precursor based electrodes for OFET applications, Adv. Funct. Mater. 17 (2007) 3111-3118. |
| [6] | I. Sondi, B. Salopek-Sondi, Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria, J. Colloid Interface Sci. 275 (2004) 177-182. |
| [7] | F.L. Xue, Z.C. Liu, Y. Su, K. Varahramyan, Inkjet printed silver source/drain electrodes for low-cost polymer thin film transistors, Microelectron. Eng. 83 (2006) 298-302. |
| [8] | Y. Shiraishi, N. Toshima, Oxidation of ethylene catalyzed by colloidal dispersions of poly(sodium acrylate)-protected silver nanoclusters, Colloids Surf. A 169 (2000) 59-66. |
| [9] | J.H. Crabtree, R.J. Burchette, R.A. Siddiqi, et al., The efficacy of silver-ion implanted catheters in reducing peritoneal dialysis-related infections, Perit Dial Int. 23 (2003) 368-374. |
| [10] | J.S. Kim, E. Kuk, K.N. Yu, et al., Antimicrobial effects of silver nanoparticles, Nanomed. Nanotechnol. Biol. Med. 3 (2007) 95-101. |
| [11] | C. Aymonier, U. Schlotterbeck, L. Antonietti, et al., Hybrids of silver nanoparticles with amphiphilic hyper branched macromolecules exhibiting antimicrobial properties, Chem. Commun. 24 (2002) 3018-3019. |
| [12] | T. Klaus, R. Joerger, E. Olsson, C.G. Granqvist, Silver-based crystalline nanoparticles, microbially fabricated, Proc. Natl. Acad. Sci. U. S. A. 96 (1999) 13611-13614. |
| [13] | S.C. Tang, X.K.Meng, H.B. Lu, S.P. Zhu, PVP-assisted sonoelectrochemical growth of silver nanostructures with various shapes, Mater. Chem. Phys. 116 (2009) 464-468. |
| [14] | S.M. Shaban, I. Aiad, M.M. El-Sukkary, E.A. Soliman, M.Y. El-Awady, One step green synthesis of hexagonal silver nanoparticles and their biological activity, J. Ind. Eng. Chem. 20 (2014) 4473-4481. |
| [15] | A. Henglein, Colloidal silver nanoparticles: photochemical preparation and interaction with O2, CCl4, and some metal ions, Chem. Mater. 10 (1998) 444-450. |
| [16] | I. Aiad, M.M. El-Sukkary, E.A. Soliman, M.Y. El-Awady, S.M. Shaban, In situ and green synthesis of silver nanoparticles and their biological activity, J. Ind. Eng. Chem. 20 (2014) 3430-3439. |
| [17] | A. Taleb, C. Petti, M.P. Pileni, Synthesis of highly monodisperse silver nanoparticles from AOT reverse micelles: a way to 2D and 3D self-organization, Chem. Mater. 9 (1997) 950-959. |
| [18] | M. Tsuji, Y. Nishizawa, K. Matsumoto, et al., Rapid synthesis of silver nanostructures by using microwave-polyol method with the assistance of Pt seeds and polyvinylpyrrolidone, Colloids Surf. A 293 (2007) 185-194. |
| [19] | G.A. Bhaduri, R. Little, R.B. Khomane, et al., Green synthesis of silver nanoparticles using sunlight, J. Photochem. Photobiol. A 258 (2013) 1-9. |
| [20] | S.M. Shaban, I. Aiad, M.M. El-Sukkary, E.A. Soliman, M.Y. El-Awady, Synthesis, surface, thermodynamic properties and biological activity of dimethylaminopropylamine surfactants, J. Ind. Eng. Chem. 20 (2014) 4194-4201. |
| [21] | Y.C. Lu, K.S. Chou, A simple and effective route for the synthesis of nano-silver colloidal dispersions, J. Chin. Inst. Chem. Eng. 39 (2008) 673-678. |
| [22] | D. Spadaro, E. Barletta, F. Barreca, G. Currò, F. Neri, PMA capped silver nanoparticles produced by UV-enhanced chemical process, Appl. Surf. Sci. 255 (2009) 8403-8408. |
| [23] | Z.L. Yang, D.D. Zhai, X. Wang, J. Wei, In situ synthesis of highly monodispersednonaqueous small-sized silver nano-colloids and silver/polymer nanocomposites by ultraviolet photopolymerization, Colloids Surf. A 448 (2014) 107-114. |
| [24] | D.N. Muanza, B.W. Kim, K.L. Euler, L. Williams, Antibacterial and antifungal activities of nine medicinal plants from zaire, Int. J. Pharm. 32 (1994) 337-345. |
| [25] | ASTM D4412-84, Standard Test Methods for Sulfate-Reducing Bacteria in Water and Water-Formed Deposits, 2009. |
| [26] | D.V. Goia, Preparation and formation mechanisms of uniform metallic particles in homogeneous solutions, J. Mater. Chem. 14 (2004) 451-458. |
| [27] | M.H. El-Rafie, M.E. El-Naggar, M.A. Ramadan, et al., Environmental synthesis of silver nanoparticles using hydroxypropyl starch and their characterization, Carbohydr. Polym. 86 (2011) 630-635. |
| [28] | J. Belloni, M. Mostafavi, H. Remita, J.L. Marignier, M.O. Delcourt, Radiationinduced synthesis of mono- and multi-metallic clusters and nanocolloids, New J. Chem. 22 (1998) 1239-1255. |
| [29] | K. Yvon, W. Jeitschko, E. Parthé, LAZYPULVERIX, a computer program, for calculating X-ray and neutron diffraction powder patterns, J. Appl. Crystallogr. 10 (1977) 73-74. |
| [30] | M.G. Guzman, J. Dille, S. Godet, Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity, World Acad. Sci. Eng. Technol. 43 (2008) 357-364. |
| [31] | R. Maity, U.N. Maiti, M.K. Mitra, K.K. Chattopadhyay, Synthesis and optical characterization of polymer-capped nanocrystalline ZnS thin films by chemical process, Physica E 33 (2006) 104-109. |
| [32] | K. Esumi, A. Suzuki, N. Aihara, K. Usui, K. Torigoe, Preparation of gold colloids with UV irradiation using dendrimers as stabilizer, Langmuir 14 (1998) 3157-3159. |
| [33] | A. Henglein, Physicochemical properties of small metal particles in solution: "microelectrode" reactions, chemisorption, composite metal particles, and the atom-to-metal transition, J. Phys. Chem. 97 (1993) 5457-5471. |
| [34] | N. Singh, P.K. Khanna, In situ synthesis of silver nano-particles in polymethylmethacrylate, Mater. Chem. Phys. 104 (2007) 367-372. |
| [35] | L.C. Courrol, F.R. De Oliveira Silva, L. Gomes, A simple method to synthesize silver nanoparticles by photo-reduction, Colloids Surf. A 305 (2007) 54-57. |
| [36] | M.V. Roldá n, L.B. Scaffardi, O. De Sanctis, N. Pellegri, Optical properties and extinction spectroscopy to characterize the synthesis of amine capped silver nanoparticles, Mater. Chem. Phys. 112 (2008) 984-990. |
| [37] | Y. Zhang, M. Yang, N.G. Portney, et al., Zeta potential: a surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells, Biomed. Microdevices 10 (2008) 321-328. |
| [38] | J. Ho, M.K. Danquah, H.T. Wang, G.M. Forde, Protein loaded mesoporous silica spheres as a controlled delivery platform, J. Chem. Technol. Biotechnol. 83 (2008) 351-358. |
| [39] | J. Hedberg, M. Lundin, T. Lowe, et al., Interactions between surfactants and silver nanoparticles of varying charge, J. Colloid Interface Sci. 369 (2012) 193-201. |
| [40] | B.H. Loo, Enhanced Raman spectroscopic study of interactions of tetracyanoethylene molecules with copper surfaces, J. Mol. Struct. 661-662 (2003) 451-457. |
| [41] | N.B. Colthup, L.H. Daly, S.E. Wiberly, Introduction to Infrared and Raman Spectroscopy, 3rd ed., Academic Press, San Diego, 1990. |
| [42] | R. Janardhanan, M. Karuppaiah, N. Hebalkar, T.N. Rao, Synthesis and surface chemistry of nano silver particles, Polyhedron 28 (2009) 2522-2530. |
| [43] | R.A. Prabu, A.P. Rajan, Review on the therapeutic potential of Vitex negundo Linn, J. Pharm. Res. 3 (2010) 1920-1922. |
| [44] | P. Balgavý, F. Devínsky, Cut-off effects in biological activities of surfactants, Adv. Colloid Interface Sci. 66 (1996) 23-63. |
| [45] | J. Pernak, J. Kalewska, H. Ksyciń ska, J. Cybulski, Synthesis and anti-microbial activities of some pyridinium salts with alkoxymethyl hydrophobic group, Eur. J. Med. Chem. 36 (2001) 899-907. |
| [46] | G. Viscardi, P.L. Quagliotto, C. Barolo, et al., Synthesis and surface and antimicrobial properties of novel cationic surfactants, J. Org. Chem. 65 (2000) 8197-8203. |
| [47] | H. Nagamune, T. Maeda, K. Ohkura, et al., Evaluation of the cytotoxic effects of bisquaternary ammonium antimicrobial reagents on human cells, Toxicol. In Vitro 14 (2000) 139-147. |
| [48] | C. Campanac, L. Pineau, A. Payard, G. Baziard-Mouysset, C. Roques, Interactions between biocide cationic agents and bacterial biofilms, Antimicrob. Agents Chemother. 46 (2002) 1469-1474. |
| [49] | M.J. Pringle, K.B. Brown, K.W. Miller,Can the lipid theories of anesthesia account for the cutoff in anesthetic potency in homologous series of alcohols? Mol. Pharmacol. 19 (1981) 49-55. |
| [50] | A.S. Janoff, M.J. Pringle, K.W. Miller, Correlation of general anesthetic potency with solubility in membranes, Biochim. Biophys. Acta 649 (1981) 125-128. |
 2015, Vol.26
2015, Vol.26