The chemistry of pyrazole derivatives has received considerable attention in the medical research due to the various biological and pharmacological properties such as antitumor [1],antimicrobial [2],anti-inflammatory [3],antiviral [4],antifungal [5],analgesic [6],and anti-hyperglycemic activity [7]. The pyrazole motif makes up the core structure of numerous biologically active compounds. One area that has been intensely studied is their anticancer activity [8]. In order to find better antitumor agents,a large number of pyrazole derivatives were synthesized and examined over the past several years [9, 10, 11, 12, 13, 14, 15]. So the use of this pharmacophore is still very powerful [16, 17].
An increase in regulatory restrictions on the use,manufacture and disposal of organic solvents has motivated the development of non-hazardous options for the development of green chemical processes. Thereby,the search for catalyst-free synthetic methods accompanied by the substitution of volatile organic solvents with sustainable media has attracted considerable interest from chemists. Moreover,the development of methods focusing on environmentally benign reactions has become particularly prominent [18, 19, 20]. In this context,ultrasound irradiation has been employed as a promoter of a wide range of synthetically useful reactions,not only because it can easily promote organic transformations,but also because it offers rapidity and high reaction yields.
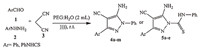
Considering the usefulness and efficiency of ultrasonic irradiation and in the course of our previous investigations on the development of catalyst-free procedures [21, 22],we report herein, an efficient method for the synthesis of highly functionalized pyrazole derivatives using ultrasound as an energy source in aqueous PEG medium without the use of any catalyst. This system can cause rapid synthesis of highly functionalized pyrazole derivatives to show the versatility of this method (Scheme 1). In continuation,a simple and an efficient one-pot synthetic approach was used for the preparation of biologically interesting novel pyrazoles derivatives with thioamide groups in good yields by means of three-component reactions of accessible starting materials such as 4-phenyl thiosemicarbazide,malononitrile, and arylaldehydes.
|
Download:
|
| Scheme 1. The represented methodology. | |
General procedure for the one-pot synthesis of pyrazole derivatives (4a-4m and 5a-5e): A mixture of aldehyde (1 mmol), malononitrile (1 mmol),phenylhydrazine or 4-phenylthiosemicarbazide (1 mmol) and 2 mL PEG: H2O (1:1) was irradiated under ultrasonic irradiation at ambient temperature for an appropriate period of time (Tables 1 and 2). After completion of the reaction (monitored by TLC analysis),the reaction mixture was diluted with water and the solid product was filtered and washed thoroughly with hot water for 20 min. Then,the crude product was purified by washing with water or crystallized from ethanol or EtOH:H2O. Caution: for long reactions,the temperature of ultrasonic bath was kept at room temperature using an ice bath.
3. Results and discussionWith this goal in mind,our initial studies of the synthesis of 5- amino-1,3-diphenyl-1H-pyrazole-4-carbonitrile 4a took advantage of the ultrasound promotion in variety of green solvents. Thus, benzaldehyde (1 mmol),malononitrile (1 mmol),phenylhydrazine (1 mmol) and an appropriate solvent were added to a flask and the mixture was sonicated and the progress of the reaction was monitored by TLC analysis. Then the final product,4a,was separated from the reaction mixture and characterized. The best result was observed when PEG-400 was used as a solvent. Water was used as a co-solvent in the method development due to its environmentally benign nature. To our surprise,when water was used as a co-solvent,the corresponding product was obtained quantitatively in short reaction time. The effect of activation technique was also investigated. In the absence of ultrasonic irradiation,the reaction was successful and the corresponding product was obtained in 78% yield,but the use of ultrasound led to better yields.
The chemical effects of ultrasonic have been attributed to the cavitation phenomena. Cavitation is a process that generates localized microscopic ‘‘hot spots’’ with transient high temperature and pressure to induce favorable conditions for the reaction [23]. In some cases sonication can also provide more efficient stirring for the reaction [24].
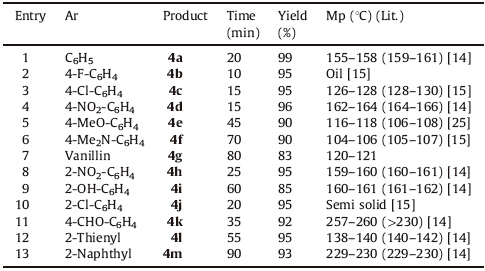
In order to demonstrate the superiority of this green methodology,the optimum condition was extended for the onepot synthesis of highly substituted pyrazole derivatives. We attempted to study the behavior of different aldehydes in the reaction with phenyl hydrazine and malononitrile under the optimum conditions. In general,aromatic aldehydes with electrondonating or electron- withdrawing groups,strically bulky aldehydes (Table 1,entry 13),heteroaryl aldehydes (Table 1,entry 12) and terephthaldehyde (Table 1,entry 11) reacted smoothly with malononitrile and phenylhydrazine to generate pyrazole derivatives in good to excellent yield. However,as depicted in Table 1,the aldehydes bearing electron-withdrawing functional groups required shorter reaction time in comparison to those having an electron-donating substituent. All the reaction under ultrasonic irradiation at room temperature completed in 10-90 min and the isolated products were respectful (Table 1).
| Table 1 Synthesis of pyrazole derivatives. |
As mentioned in Table 1,the results clearly indicate that the reaction proceeded in high yields and produced the desirable products. Notably,the reactions were clean and all the pure products were obtained after filtration and washing with water.
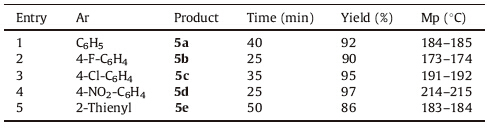
Substituted pryazole derivatives played an important role in a variety of biological activities [17]. Surprisingly,we found that our catalyst-free system demonstrated excellent tolerance in the synthesis of pyrazole carbothioamide derivatives. The onepot, three-component ultrasonic irradiated reaction involving arylaldehydes,malononitrile and 4-phenylthiosemicarbazide proceeded at room temperature in PEG 400:H2O (2 mL),to give 5- amino-3-aryl-4-cyano-N-phenyl-1H-pyrazole-1-carbothioamide derivatives (5a-5e) as the only product in short reaction time and high yields. The products were fully characterized on the basis of spectroscopic data (see Supporting information). It is worth mentioning that all the pyrazole carbothioamide derivatives that synthesized by this method precipitated from the reaction mixture and can be purified easily by recrystallization from the ethanol (Table 2).
| Table 2 Synthesis of 5-amino-3-aryl-4-cyano-N-phenyl-1H-pyrazole-1-carbothioamide derivatives (5a–5e). |
Based on the reported literatures [15, 26],we propose a plausible mechanism for the synthesis of pyrazole derivatives as depicted in Scheme 2. The mixture of PEG 400 and water helped the enolization of malononitrile and increased the nucleophilic character of the methylene carbon through hydrogen bonds [21]. As a result,the Knoevenagel condensation and Michael addition produced intermediates A and B,respectively. Phenyl hydrazine functioned as both a Brønsted base and a nucleophile in this catalyst free system [18]. Subsequently,annulation,tautomerization and aromatization of the intermediate B yielded the final product.
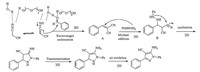
|
Download:
|
| Scheme 2. A plausible mechanism. | |
We have also successfully applied this new methodology on a larger scale. For example up to 15 mmol of benzaldehyde, 4-phenylthiosemicarbazide and malononitrile could produce 5- amino-3-aryl-4-cyano-N-phenyl-1H-pyrazole-1-carbothioamide (5a) in a yield of up to 80% under these reaction conditions.
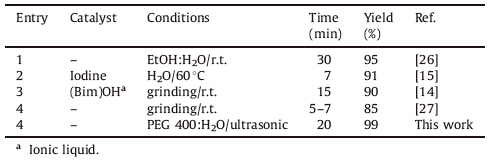
To highlight the merit of this method,Table 3 shows the comparison of the efficiency of synthesis of pyrazole derivatives using our method with some of those reported in the literatures. Each of these methods has their own advantages,but using of mixture of PEG 400 and water without a catalyst as shown in this protocol could be a new alternative in the economical synthesis of highly substituted pyrazoles.
| Table 3 Comparison of result using our reaction system with results obtained by other published works for the synthesis of 5-amino-1,3-diphenyl-1H-pyrazole-4- carbonitrile (4a). |
In summary,we have developed an expedient and clean method for the synthesis of highly substituted pyrazoles via easily accessible starting materials. Using a mixture of PEG:H2O without any catalyst makes this protocol an interesting alternatives to the traditional reaction systems. The simplicity of the methodology, ease of the product isolation and mild conditions make this process suitable on an industrial scale.
AcknowledgmentThe authors gratefully acknowledge Semnan University Research Council for financial support of this work.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.07. 009.
| [1] | S. Manfredini, R. Bazzanini, P.G. Baraldi, et al., Pyrazole-related nucleosides. 4. Synthesis and antitumor activity of some 1-tetrahydropyranyl-4-substituted pyrazoles, Anti-cancer Drug Des. 11 (1996) 193–204. |
| [2] | A.A. Bekhit, H.T.Y. Fahmy, S.A.F. Rostom, A.M. Baraka, Design and synthesis of some substituted 1H-pyrazolyl-thiazolo[4,5-d]pyrimidines as anti-inflammatory- antimicrobial agents, Eur. J. Med. Chem. 38 (2003) 27–36. |
| [3] | S.G. Alegaon, K.R. Alagawadi, M.K. Garg, K. Dushyant, D. Vinod, 1,3,4-Trisubstituted pyrazole analogues as promising anti-inflammatory agents, Bioorg. Chem. 54 (2014) 51–59. |
| [4] | O.I. El-Sabbagh, M.M. Baraka, S.M. Ibrahim, et al., Synthesis and antiviral activity of new pyrazole and thiazole derivatives, Eur. J. Med. Chem. 44 (2009) 3746–3753. |
| [5] | R. Sridhar, P.T. Perumal, S. Etti, et al., Design, synthesis and anti-microbial activity of 1H-pyrazole carboxylates, Bioorg. Med. Chem. Lett. 14 (2004) 6035–6040. |
| [6] | G. Menozzi, L. Mosti, P. Fossa, F. Mattioli, M. Ghia, v-Dialkylaminoalkyl ethers of phenyl-(5-substituted 1-phenyl-1H-pyrazol-4-yl)methanols with analgesic and anti-inflammatory activity, J. Heterocycl. Chem. 34 (1997) 963–968. |
| [7] | G.R. Bebernitz, G. Argentieri, B. Battle, et al., The effect of 1,3-diaryl-[1H]- pyrazole-4-acetamides on glucose utilization in ob/ob mice, J. Med. Chem. 44 (2001) 2601–2611. |
| [8] | A.M. Mohamed, W.A. El-Sayed, M.A. Alsharari, et al., Anticancer activities of some newly synthesized pyrazole and pyrimidine derivatives, Arch. Pharm. Res. 36 (2013) 1055–1065. |
| [9] | H.F. Zhang, Z.Q. Ye, G. Zhao, Enantioselective synthesis of functionalized fluorinated dihydropyrano [2,3-c]pyrazoles catalyzed by a simple bifunctional diaminocyclohexane- thiourea, Chin. Chem. Lett. 25 (2014) 535–540. |
| [10] | D.V. Jawale, U.R. Pratap, J.R. Mali, R.A. Mane, Silica chloride catalyzed one-pot synthesis of fully substituted pyrazoles, Chin. Chem. Lett. 22 (2011) 1187– 1190. |
| [11] | C. Wang, Y.H. Jiang, C.G. Yan, Convenient synthesis of spiro[indoline-3,40- pyrano[2,3-c]pyrazole] and spiro[acenaphthyl-3,40-pyrano[2,3-c]pyrazoles] via four-component reaction, Chin. Chem. Lett. 26 (2015) 889–893. |
| [12] | Y. Li, C.E. Dong, Efficient synthesis of fused pyrazoles via simple cyclization of o-alkynylchalcones with hydrazine, Chin. Chem. Lett. 26 (2015) 623–626. |
| [13] | F. Moeinpour, A. Khojastehnezhad, Cesium carbonate supported on hydroxyapatite coated Ni0.5Zn0.5Fe2O4 magnetic nanoparticles as an efficient and green catalyst for the synthesis of pyrano[2,3-c]pyrazoles, Chin. Chem. Lett. 26 (2015) 575–579. |
| [14] | M. Srivastava, P. Rai, J. Singh, J. Singh, Efficient iodine-catalyzed one pot synthesis of highly functionalised pyrazoles in water, New J. Chem. 38 (2014) 302–307. |
| [15] | M. Srivastava, P. Rai, J. Singh, J. Singh, An environmentally friendlier approachionic liquid catalysed, water promoted and grinding induced synthesis of highly functionalised pyrazole derivatives, RSC Adv. 3 (2013) 16994–16998. |
| [16] | H.A.H. Shamroukh, A.E. Rashad, E. Abdel-Megeid, H.S. Ali, M.M. Ali, Some pyrazolo[3,4-d] pyrimidine derivatives: synthesis and anticancer evaluation, Arch. Pharm. 347 (2014) 559–565. |
| [17] | M. Tang, F.M. Zhang, Efficient one-pot synthesis of substituted pyrazoles, Tetrahedron 69 (2013) 1427–1433. |
| [18] | Y. Gu, Multicomponent reactions in unconventional solvents: state of the art, Green Chem. 14 (2012) 2091–2128. |
| [19] | S. Gaddam, H.R. Kasireddy, K. Konkala, et al., Synthesis of N-substituted-2- aminobenzothiazoles using nano copper oxide as a recyclable catalyst under ligand-free conditions, in reusable PEG-400 medium, Chin. Chem. Lett. 25 (2014) 732–736. |
| [20] | J. Sindhu, H. Singh, J.M. Khurana, C. Sharma, K.R. Aneja, Multicomponent domino process for the synthesis of some novel 5-(arylidene)-3-((1-aryl-1H-1,2,3-triazol- 4-yl)methyl)-thiazolidine-2,4-diones using PEG-400 as an efficient reaction medium and their antimicrobial evaluation, Chin. Chem. Lett. 26 (2015) 50–54. |
| [21] | F. Nemati, H. Kiani, A green and highly efficient protocol for catalyst-free Knoevenagel condensation and Michael addition of aromatic aldehydes with 1,3-cyclic diketones in PEG-400, Chin. J. Chem. 29 (2011) 2407–2410. |
| [22] | F. Nemati, M.M. Hosseini, H. Kiani, Glycerol as a green solvent for efficient, onepot and catalyst free synthesis of 2,4,5-triaryl and 1,2,4,5-tetraaryl imidazole derivatives, J. Saudi Chem. Soc. (2013), http://dx.doi.org/10.1016/j.jscs.2013.02. 004. |
| [23] | R. Cella, H.A. Stefani, Ultrasound in heterocycles chemistry, Tetrahedron 65 (2009) 2619–2641. |
| [24] | Y. Zou, Y. Hu, H. Liu, D. Shi, Rapid and efficient ultrasound-assisted method for the combinatorial synthesis of spiro[indoline-3,40-pyrano[2,3-c]pyrazole] derivatives, ACS Comb. Sci. 14 (2012) 38–43. |
| [25] | K.A. Kumar, P. Jayaroop, Synthesis, characterization and chelating properties of novel heterocyclic azo dyes containing ligand, Int. J. Pharm. Tech. Res. 5 (2014) 364–368. |
| [26] | A. Hasaninejad, S. Firoozi, Catalyst-free, one-pot, three-component synthesis of 5-amino-1,3-aryl-1H-pyrazole-4-carbonitriles in green media, Mol. Divers. 17 (2013) 459–469. |
| [27] | P.S. Bhale, S.B. Dongare, U.B. Chanshetti, Simple grinding, catalyst-free, one-pot, three-component synthesis of polysubstituted amino pyrazole, Res. J. Chem. Sci. 4 (2014) 16–21. |
 2015, Vol.26
2015, Vol.26