b School of Pharmaceutical Sciences, Nanjing Tech University, Nanjing 211816, China;
c State Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing Tech University, Nanjing 211816, China
Polyamide,commercially named Nylon by DuPont,has been identified as a crucial class of engineering plastics since 1930s [1- 3]. Polyamide 6 (PA6 or Nylon 6),one of the biggest branches of polyamide,is broadly used in fibers,autos and composite materials for the outstanding mechanical properties,corrosion resistance, chemical inertness and abrasion performance [4, 5].
In contrast to the traditional commercial linear PA6 product, star-branched polyamide 6 (SPA6) has drawn the academic and industrial interest for the increased performance with potential advantages in engineering applications [6, 7, 8, 9]. Various synthetic routes to SPA6 have been established through different polymerization mechanisms. Cationic ring-opening polymerization (ROP) of e-caprolactam(CL) with starburst poly(ethylenimine) as an initiator was used to prepare six-arm SPA6 [10]. The resultant polymer showed decreased viscosity in the melt and in solution and retained mechanical properties. Dentritic polyamide 6 wasreported utilizing a novel third generation polyamidoamine (PAMAM) dendrimer initiator [11]. Hydrolytic ROP was the main method to produce PA6 in industry. Three-arm SPA6 was generated in the presence of trimesinicacid through a hydrolytic mechanism [12, 13]. However, the reaction conditions required long reaction time (>2 h),high temperature (>200 ℃) and pressure (>1 MPa) for both cationic and hydrolytic polymerizations.
The anionic ROP of CL is an attractive protocol to melting cast polyamide 6 (MC PA6) in industry owing to the shorter reaction time (10-30 min),lower reaction temperature (150-180 ℃) and pressure (atm) [14, 15, 16]. The anionic polymerization was usually conducted by a two-component system of a catalyst and an Nacyllactam type activator. The growing chain ends are always corresponding to N-acyllactam and the number of the polymer chains should be equal to that of the N-acyllactam if there are no side reactions. Thus,non-linear PA6 could be theoretically fabricated by adding multifunctional N-acyllactam type activators [17, 18]. Ashida et al. explored a tertiary amine centered trifunctional activator from Desmodur L-2291A and CL [6]. Santos et al. synthesized a star-like PA6-block-polyurethane copolymer using a tri-arm polyurethane activator with glycol as a core moiety [19]. They found that the star-like copolymer showed better mechanical properties compared with the linear ones. However,these multifunctional activators suffered from the tedious synthesis and toxic isocyanate reagent. There is a great need to systematically investigate simple and novel multifunctional activators and SPA6.
Inspired by the pioneering work about SPA6 and anionic polymerization of CL,we proposed a simple isocyanate-free, benzene-centered trifunctional activator of N,N' ,N''-trimesoyltricaprolactam. SPA6 was successfully synthesized via CL anionic polymerization in the presence of this multifunctional activator. The structure and properties of the product was studied using the relative viscosity test,FTIR,DSC and XRD analysis.
2. Experimentalε-Caprolactam (CL) (Sinopharm Chemical Reagent,AR) was dried under vacuum at 40 ℃ for 12 h and stored under N2. Tetrahydrofuran (THF) (Sinopharm Chemical Reagent,>99%) was distilled over sodium under nitrogen atmosphere. 1,3,5-Benzoyl chloride (J&K,99%),n-butyl lithium (J&K,2.4 mol/L solution in hexane),N-acetyl-ε-caprolactam (AC) (TCI,>99%),ε-caprolactam magnesium bromide (CL-Mg-Br) (Brüggolen,>99%) and other chemicals were used without purification.
N,N' ,N''-trimesoyltricaprolactam (TMTC) was synthesized according to the previous literature [20]. To a solution of CL (2.441 g,0.0216 mol) in dry THF (100 mL) was added n-butyl lithium (10.0 mL,0.0240 mol) dropwise at -78 ℃. After 30 min of stirring,1,3,5-benzoyl chloride (1.720 g,0.0065 mol) in dry THF (30 mL) was added dropwise to the solution. Subsequently the reaction was carried out at 25 ℃ for 12 h before quenching by water. Aqueous layer was extracted with ethyl acetate and the combined organic extracts were washed with brine,dried over MgSO4 overnight,filtered and concentrated. The product was afforded after flash chromatography (petroleum ether/ethyl acetate: 5/1-5/5) and stored under N2 (Yield 65.6%). 1H NMR (CDCl3): δ 7.66 (s,3H,Ar),3.93 (m,6H,-CO-CH2-CH2-CH2-CH2- CH2-),2.69 (m,6H,-CO-CH2-CH2-CH2-CH2-CH2-),1.64 (m,18H, -CO-CH2-CH2-CH2-CH2-CH2-). HRMS: m/z 518.248 (M+Na+).
The typical anionic polymerization of CL for SPA6 and LPA6 was conducted as the follows. Weighted dry CL (11.307 g,0.100 mol), CL-Mg-Br (0.645 g,0.003 mol) and TMTC (0.495 g,0.001 mol) were added into an ampoule under nitrogen atmosphere. The polymerization was conducted for 15 min at 160 ℃. The raw product was extracted with freshly distilled THF for 48 h and dried to the constant weight (Yield 96.7%).
1H NMR (400 MHz) spectra were recorded on a Bruker-400 spectrometer in CDCl3 with tetramethylsilane as the internal reference. High resolution mass spectra (HRMS) were performed on an Agilent Q TOF 6520 mass spectrometer with electron spray ionization (ESI) as the ionization mode. Relative viscosity (RV) was tested using an Ubbelohde viscometer at 1 g/100 mL in 98% sulfuric acid at 25 ± 0.01 ℃. Fourier transforminfrared spectroscopy (FTIR) was measured on a Nicolet IS5 FT-IR. 32 scans were made and 4000 cm-1 to 400 cm-1 spectra were analyzed. Differential scanning calorimetry (DSC) was performed on a DSC 823e differential scanning calorimeter from Mettler Doritos. Samples were heated from 25 ℃ to 250 ℃ in the first scan at 10 ℃/min. After cooling to 25 ℃ at 10 ℃/min,the sample was heated up to 250 ℃ at 10 ℃/min for a second scan. X-ray Diffraction (XRD) was carried out on a Smartlab instrument at 35 kV and 25 mA. The data were collected from 10° to 40° (2θ) with a scanning velocity of 1°/min.
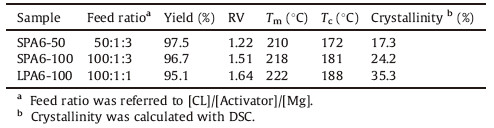
3. Results and discussionStar-branched polyamide 6 (SPA6) could be synthesized in the presence of a two-component system of a catalyst and a multifunctional activator. Herein,we synthesized a simple benzene-centered trifunctional activator of N,N' ,N''-trimesoyltricaprolactam (TMTC) from 1,3,5-benzoyl chloride and CL (Scheme 1). The yield was above 60% under mild conditions. The structure of TMTC was characterized with NMR and HRMS. ε-Caprolactam magnesium bromide (CL-Mg-Br) was chosen to catalyze the CL anionic ROP due to its high catalytic activity [21]. The polymerization results were summarized in Table 1. The starbranched samples with [CL]/[TMTC] feed ratio of 50 and 100 were obtained and termed as SPA6-50 and SPA6-100 respectively. As the control,linear polyamide 6 (LPA6) was prepared with a commercial monofunctional activator (N-acetyl-e-caprolactam,AC). LPA6- 100 was referred to the linear counterpart with the same [CL]/[AC] feed ratio of 100. The yields of over 95% for both SPA6 and LPA6 were achieved at 160 ℃ for 15 min.

|
Download:
|
| Scheme. 1.Synthesis of N,N' ,N''-trimesoyltricaprolactam and SPA6. | |
| Table 1 Results of SPA6 and LPA6 via anionic polymerization. |
As we known,the performances of star-branched polymers differ from their linear counterparts. Therefore,the existence of a star-branched structure could be demonstrated by comparison of the properties [6, 10, 11, 12, 13, 19]. The molecular weight of SPA6 and LPA6 was measured with the relative viscosity (RV) test. The RV values of SPA6 increased from 1.22 (SPA6-50) to 1.51 (SPA6-100) as the [CL]/[TMTC] feed ratio increased. LA6-100 exhibited a larger RV (1.64) than SPA6-100. The previous calculations and experiments showed that the mean square radius gyration of a branched polymer was smaller than that of a linear sample with similar molecular weight [10]. The difference in RV indicated the formation of a star-branched structure.
The chemical structure of SPA6 was characterized with FTIR. As depicted in Fig. 1,SPA6-100 and LPA6-100 exhibited almost the same featured bands. The characteristic band at 1640 cm-1 was assigned to the amide C55O stretch. The appearance of N-H stretch at 3300 cm-1,C-N bending at 1548 cm-1,torsion in hydrogen bond at 584 cm-1 was clearly shown. It was noteworthy that less intense characteristic bands of SPA6-100 were observed. The stacking of the polymer chains was disturbed by the branched points. The hydrogen bonding interactions between neighboring chains were reduced so that the less intense FTIR bands of SPA-100 were detected with comparison of LPA6-100 [13, 19].

|
Download:
|
| Fig. 1.FTIR of SPA6 and LPA6. | |
The thermal properties were examined with DSC. The previous thermal history of the specimen was removed by heating it to above its melting point and subsequently cooling it down to room temperature. As illustrated in Fig. 2,an endothermic peak at 222 ℃ assigned to the Tm was observed in sample LPA6-100. In contrast, SPA6-100 exhibited a decreased Tm at 218 ℃. Moreover,the crystallinity of SPA6-100 (ΔHf = 45.98 J/g) was calculated to be 24.2% (ΔHf 0 = 190 J/g),which was lower than that of LPA6-100 (DHf = 67.11 J/g) [15]. The crystallization temperature (Tc) of starbranched and linear samples demonstrated the same tendency. The decreased Tm,Tc and crystallinity would offer potential advantages in material processing and elucidate the existence of branched structures,which could disturb the original arrangement and hydrogen bonding interactions of the polymer chains [12, 19].

|
Download:
|
| Fig. 2.DSC of SPA6 and LPA6. | |
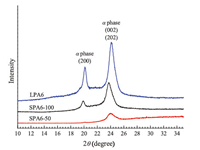
The crystal structures of PA6 were determined with XRD. In Fig. 3,LPA6 showed the characteristic peaks at 2θ = 20.4° and 24.0° which were associated with the monoclinic structure (α phase) [15]. The diffraction curve intensity of SPA6-100 and SPA6-50 dropped. It meant that the crystallinity decreased as the number of branched points grew,which was consistent with the results of DSC [19, 22].
RV,FTIR,DSC and XRD analyses provided indirect evidence to confirm the existence of a star-branched structure according to the pioneer work about star-branched polyamide 6 [6, 10, 11, 12, 13, 19]. The direct proof such as NMR and MALDI TOF MS was still challenging due to the low solubility and high viscosity of SPA6 [23]. In the future,we will further investigate the detailed structure characterization and mechanical properties [24].
|
Download:
|
| Fig. 3.XRD of SPA6 and LPA6. | |
In this article,we synthesized a simple benzene-centered trifunctional activator of N,N' ,N''-trimesoyltricaprolactam. Subsequently, star-branched polyamide 6 was prepared via ε-caprolactam magnesium bromide catalyzed anionic ring-opening polymerization of ε-caprolactam in the presence of this activator. In contrast to its linear counterparts,the resultant star-branched polyamide 6 showed smaller relative viscosity,decreased melting temperature and lower crystallinity. The specific properties demonstrated the existence of a star-branched structure and provided potential advantages in engineering applications.
AcknowledgmentThis work was supported by a grant from the National High Technology Research and Development Program of China (No. 2014AA021201),the National Basic Research Program of China (No. 2012CB721104),China Postdoctoral Science Foundation (No. 2014M551574) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
| [1] | F. Jia, J.L. Mao, X.Y. Yang, Y. Ma, C. Yao, Thermal, physical and mechanical properties of hydrogenated dimer acid-based Nylon 636/Nylon 66 copolymers, Chin. Chem. Lett. 24 (2013) 654-658. |
| [2] | Z. Xu, C. Gao, In situ polymerization approach to graphene-reinforced nylon-6 composites, Macromolecules 43 (2010) 6716-6723. |
| [3] | M. Ghaemy, H. Behmadi, R. Alizadeh, Synthesis of organosoluble polyamides with bulky triaryl imidazole pendent group, Chin. Chem. Lett. 20 (2009) 961-964. |
| [4] | S. Naumann, S. Epple, C. Bonten, M.R. Buchmeiser, Polymerization of ε-caprolactam by latent precatalysts based on protected N-heterocyclic carbenes, ACS Macro Lett. 2 (2013) 609-612. |
| [5] | Z.Y. Wu, W. Xu, J.K. Xia, et al., Flame retardant polyamide 6 by in situ polymerization of e-caprolactam in the presence of melamine derivatives, Chin. Chem. Lett. 19 (2008) 241-244. |
| [6] | W.L. Chang, K.C. Frisch, K. Ashida, Anionic polymerization of star-shaped nylon 6 with a trifunctional initiator, J. Polym. Sci. A: Polym. Chem. 27 (1989) 3637-3649. |
| [7] | K. Miyata, Y. Watanabe, T. Itaya, T. Tanigaki, K. Inoue, Synthesis of heteroarm starshaped block copolymers with cyclotriphosphazene core and their compatibilizing effects on PPO/Nylon 6 blends, Macromolecules 29 (1996) 3694-3700. |
| [8] | N. Hasegawa, A. Usuki, A. Okada, Thermal properties of novel star-shaped nylon 6, Kobunshi Ronbushu 53 (1996) 537-541. |
| [9] | A. Usuki, N. Hasegawa, A. Okada, T. Kurauchi, Synthesis and properties of novel star-shaped nylon6, Kobunshi Ronbushu 52 (1995) 576-581. |
| [10] | J.M. Warakomski, Synthesis and properties of star-branched nylon 6, Chem. Mater. 4 (1992) 1000-1004. |
| [11] | F. Zhang, L. Zhou, Y.C. Liu, W.J. Xu, Y.Q. Xiong, High-flow nylon 6 by in situ polymerization: synthesis and characterization, J. Appl. Polym. Sci. 108 (2008) 2365-2372. |
| [12] | L.X. Dai, N.X. Huang, Z.L. Tang, K.-D. Hungenberg, Preparation and characterization of polyamide-6 with three-branched chains, J. Appl. Polym. Sci. 82 (2001) 3184-3193. |
| [13] | P. Fu, M.L. Wang, M.Y. Liu, et al., Preparation and characterization of star-shaped nylon 6 with high flowability, J. Polym. Res. 18 (2011) 651-657. |
| [14] | K. Hashimoto, Ring-opening polymerization of lactams. Living anionic polymerization and its applications, Prog. Polym. Sci. 25 (2000) 1411-1462. |
| [15] | J.C. Farias-Aguilar, M.J. Ramírez-Moreno, L. Té llez-Jurado, L.H. Balmori-Ramírez, Low pressure and low temperature synthesis of polyamide-6 (PA6) using Na0 as catalyst, Mater. Lett. 16 (2014) 388-392. |
| [16] | Y.A. Piskun, I.V. Vasilenko, L.V. Gaponik, S.V. Kostjuk, Activated anionic ringopening polymerization of ε-caprolactam with magnesium di(e-caprolactamate) as initiator: effect of magnesium halides, Polym. Bull. 68 (2012) 1501-1513. |
| [17] | T.V. Volkova, Y.S. Vygodskii, O.N. Zabegaeva, et al., Synthesis and characterization of grafted copolymers of aromatic polyimides and e-caprolactam, J. Appl. Polym. Sci. 114 (2009) 577-586. |
| [18] | Y. Pae, Preparation and characterization of polyimide-g-nylon 6 copolymers from nonfunctionalized polyimides, J. Appl. Polym. Sci. 99 (2006) 292-299. |
| [19] | E.A. González-De Los Santos, A.S. Ló pez-rodríguez, M.J. Lozano-gonzá lez, F. Soriano- Corral, Starlike Nylon 6/polyurethane block copolymers by reaction injection- molding process (RIM), J. Appl. Polym. Sci. 80 (2001) 2483-2494. |
| [20] | N. Gigant, I. Gillaizeau, Construction of nitrogen-fused tetrahydroquinolines via a domino reaction, Org. Lett. 14 (2012) 4622-4625. |
| [21] | N. Barhoumi, A. Maazouz, M. Jazir, R. Abdelhedi, Polyamide from lactams by reactive rotational molding via anionic ring-opening polymerization: optimization of processing parameters, Express Polym. Lett. 7 (2013) 76-87. |
| [22] | B.G. Risch, G.L. Wilkes, J.M. Warakomski, Crystallization kinetics and morphological features of star-branched nylon-6: effect of branch-point functionality, Polymer 34 (1993) 2330-2343. |
| [23] | E. Casazza, L. Ricco, S. Russo, E. Scamporrino, Nature of a low molar mass peak in anionic poly(ε-caprolactam). Its identification as macrocyclic ensemble, Macromolecules 40 (2007) 739-745. |
| [24] | J. Myers, Z. Chen, Surface plasma treatment effects on the molecular structure at polyimide/air and buried polyimide/epoxy interfaces, Chin. Chem. Lett. 26 (2015) 449-454. |
 2015, Vol.26
2015, Vol.26