Dimethyl carbonate (DMC) is an environmentally benign chemical product with biodegradability and low toxicity [1]. It has attracted more and more attention and widely used as the methylation or carbonylation agent, solvent, and fuel additive [2, 3,4, 5,6, 7,].
The synthesis of DMC from CO and methyl nitrite (MN) was developed by Ube Industries [8,9]. DMC is synthesized over supported palladium catalysts in the Ube process [10,11]. Among all the supported palladium catalysts, activated carbon (AC) supported Wacker-type catalysts (PdCl2–CuCl2/AC) exhibited excellent catalytic performance for the synthesis of DMC from CO and MN [12,13]. Recently, the potential of various activated carbon supported Wacker-type catalysts promoted by different alkali promoters such as potassium (K) promoters was evaluated in the synthesis of DMC by oxycarbonylation of methanol [14,15,16]. It is found that the presence of alkali promoters such as CH3COOK benefits the vapor-phase DMC synthesis reaction and the well-defined Cu2Cl(OH)3 is supposed to be the active phase [17]. The essential role of alkali promoters may be as a result of electron-donating properties of K, which has an effect upon the electronic environment of palladium and copper active species in the Wacker-type catalyst [14]. Jiang et al. [14]. have proved that the catalytic activities of activated carbon supported Wacker-type catalysts promoted by different alkali promoters ranked in the following order: K > Na > Li. And the interaction between CH3COOK and CuCl2 or PdCl2 during the preparation of catalyst resulted in the formation of KCl species which suppressed the chlorine loss of Wacker-type catalysts. However, it has not yet been reported that the effects of potassium promoters of Wacker-type catalysts on the synthesis of DMC from CO and MN.
In this paper, activated carbon supported Wacker-type catalysts promoted by different K promoters were prepared by different methods and the catalytic activities of catalysts with different mass content of K promoter were evaluated. The effect of different K promoters on the catalytic performance of PdCl2–CuCl2/AC catalysts for DMC synthesis from CO and MN was investigated to explore an effective K promoted PdCl2–CuCl2/AC catalyst for the DMC formation.
2. ExperimentalCatalyst preparation: The AC supported Wacker-type catalysts were prepared by a two-step impregnation method. 0.057 g KCl was dissolved in 5 mL distilled water and KCl aqueous solution was added drop by drop to activated carbon. After impregnated for 24 h at room temperature, the sample was dried at 373 K for 4 h and the obtained sample is labeled as AC–KCl. PdCl2–CuCl2/AC catalyst with KCl promoter prepared by the incipient wetness impregnation method (IWI) is as follows: 7 mL of methanol solution of PdCl2 and CuCl2 was added by drops to AC–KCl, impregnating at room temperature for 24 h, drying at 373 K for 4 h and calcining at 473 K for 4 h. And the obtained catalyst is labeled as IWI-PdCl2–CuCl2– KCl/AC. PdCl2–CuCl2/AC catalyst with KCl promoter prepared by the excessive impregnation method is as follows: AC–KCl was added into 50 mL of methanol solution of PdCl2 and CuCl2 and stirred for 4 h, followed by removing solvent by vacuum evaporation at 323 K, drying at 373 K for 4 h and calcining at 473 K for 4 h. And the obtained catalyst is labeled as PdCl2–CuCl2– KCl/AC. PdCl2–CuCl2/AC catalysts with different K promoters (KOH, CH3COOK and K2CO3) were also prepared by the excessive impregnation method and are labeled as PdCl2–CuCl2–KOH/AC, PdCl2–CuCl2–CH3COOK/AC and PdCl2–CuCl2–K2CO3/AC, respectively. The loadings of Cu and Pd for all catalysts were 1.2 and 1.0 wt%, respectively.
Catalyst characterization: Textural properties of different K promoters modified AC supported Wacker-type catalysts were measured with N2 adsorption and desorption on a Micromeritics ASAP 2020 apparatus at 77 K. H2-temperature-programmed reduction (H2-TPR) experiments were carried out on the Micromeritics AutoChem 2920 apparatus. The catalyst surface copper species were analyzed by X-ray photoelectron spectroscopy (XPS), which was carried out on a Perkin-Elmer PHI 1600 ESCA system.
Activity test and product analysis: The DMC synthesis from CO and MNover AC supported Wacker-type catalyst was conducted at 0.2 MPa, 393 K in a continuous flow fixed-bed reactor. 2 mL of catalyst (20–40 mesh) sample were loaded in the middle of fix-bed. The inlet gas was composed of CO, MN and N2, the volume ratio of CO/MN was 1.0. The gas hourly space velocity (GHSV) of the reaction was 4000 h-1. The products were analyzed by Agilent 4890A GC equipped with a FID detector.
3. Results and discussionFig. 1 shows the catalytic performance of PdCl2–CuCl2/AC catalysts with different K promoters, which were CH3COOK, KCl, KOHand K2CO3. FromFig. 1, it is obviously noted that the space time yield (STY) of DMC was around 900 g (L-cat)-1 h-1 over PdCl2– CuCl2–KCl/AC catalysts,while itwas only around700 g (L-cat)-1 h-1 over PdCl2–CuCl2/AC without any K promoter. Comparatively, the STY of DMC over PdCl2–CuCl2/AC catalysts promoted by KOH, CH3COOK and K2CO3 was much lower, only around 550, 400 and 200 g (L-cat)-1 h-1, respectively. The results showed that the STY of DMC and the selectivity of DMC based on MN and CO on PdCl2–CuCl2/AC catalysts with different K promoters ranked in the following order: KCl > KOH > CH3COOK > K2CO3. Especially, the addition of KCl promoter significantly improved the catalytic activities of PdCl2–CuCl2/AC catalyst for DMC synthesis from CO andMN. Therefore, the additionofKpromoters in the formofKCl can significantly enhance the catalytic activities of DMC synthesis from CO and MN.

|
Download:
|
| Fig. 1.Catalytic activities of PdCl2–CuCl2/AC catalysts with different K promoters (K loading 1.0 wt%). | |
Textural properties of activated carbon and PdCl2–CuCl2/AC catalysts with different K promoters are listed in Table 1. It is noted that the specific surface area, pore volume and average pore diameter of all the catalysts were slightly changed, suggesting that the structure and physical properties of PdCl2–CuCl2/AC catalysts with different K promoters were similar. Therefore, the different K promoters had negligible effect on the textural properties of K promoters modified PdCl2–CuCl2/AC catalysts.
| Table 1 Textural properties of the catalysts. |
H2-TPR profiles of PdCl2–CuCl2/AC catalysts with different K promoters are illustrated in Fig. 2. It can be seen that there is one reduction peak around 200 °C for all the catalysts. This reduction peak is due to the co-reduction of Cu2+ and Pd2+ species. According to previous research, it can be seen that the strong interactions among activated carbon support, PdCl2 and CuCl2 species led to the overlap of peaks of Cu2+ and Pd2+ species into one peak [18,19]. It is noted that this reduction peak was shifted to higher temperature, and the reduction temperature of all the catalysts ranked in the following order: PdCl2–CuCl2–KCl/AC < PdCl2–CuCl2–KOH/AC < PdCl2–CuCl2–CH3COOK/AC < PdCl2–CuCl2–K2CO3/AC. It is well known that Pd2+ species is the main active species of DMC synthesis from CO andMN[11]. However, Pd2+ species is easy to be reduced to Pd0 species during the reaction, resulting in the deactivation of the Wacker-type catalysts [12]. It is noted that the existence of Cu2+ species is responsible for oxidizing Pd0 to Pd2+. Thus, the higher the reduction temperature of Cu2+ species, the more difficult it is to oxidize Pd0 to Pd2+. Thus, PdCl2–CuCl2/AC catalysts with CH3COOK, KOH and K2CO3 promoters are not favorable for DMC synthesis from CO andMNbecause of the higher reduction temperature of Cu2+ species.
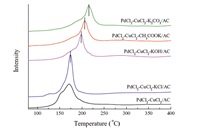
|
Download:
|
| Fig. 2.H2-TPR profiles of PdCl2–CuCl2/AC catalysts with different K promoters. | |
Fig. 3 shows XPS spectra of PdCl2–CuCl2/AC and PdCl2–CuCl2– KCl/AC catalysts before and after reaction. It can be seen that there are two peaks associated with copper species with the binding energy around 934.0 eV and 932.0 eV in all the catalysts. The former is ascribed to Cu2+ species and the latter is attributed to Cu+ species [15,20]. The area percentages of Cu 2p for all the catalysts were further analyzed. The proportion of Cu2+ is 62.8% and 79.7% for PdCl2–CuCl2/AC and PdCl2–CuCl2–KCl/AC catalysts before reaction, respectively. Meanwhile, the amount of Cu2+ in the PdCl2–CuCl2–KCl/AC catalyst after reaction is also higher than that in the PdCl2–CuCl2/AC catalyst after reaction. It can be concluded that PdCl2–CuCl2/AC catalyst promoted by KCl led to possessing larger amount of Cu2++. It is known that Cu2+ species is responsible for oxidizing Pd0 to active Pd2++ and more Cu2++ species will benefit the catalytic performance of Wacker-type catalysts for DMC synthesis from CO and MN. Therefore, PdCl2–CuCl2–KCl/AC catalyst with more Cu2++ species performed excellent catalytic activities for DMC synthesis.

|
Download:
|
| Fig. 3.XPS spectra of PdCl2–CuCl2/AC and PdCl2–CuCl2–KCl/AC catalysts before and after reaction. (a) PdCl2–CuCl2/AC (before reaction); (b) PdCl2–CuCl2/AC (after reaction);(c) PdCl2–CuCl2–KCl/AC (before reaction); (d) PdCl2–CuCl2–KCl/AC (after reaction). | |
Fig. 4 shows the catalytic performance of PdCl2–CuCl2–KCl/AC catalysts with different preparation method for DMC synthesis from CO and MN. For PdCl2–CuCl2–KCl/AC catalyst prepared by excessive impregnation method, STY of DMC reached a relative stable value of around 900 g (L-cat)-1 h-1 after 10 h reaction, while STY of DMC on IWI-PdCl2–CuCl2–KCl/AC catalyst was around 750 g (L-cat)-1 h-1. Meanwhile, for the two catalysts, the selectivity of DMC based on MN and CO was basically the same. It is suggested that the catalytic performance of PdCl2–CuCl2–KCl/AC catalyst was superior to that of IWI-PdCl2–CuCl2–KCl/AC catalyst. Therefore, PdCl2–CuCl2–KCl/AC prepared by excessive impregnation method is more favorable for the synthesis of DMC from CO and MN.

|
Download:
|
| Fig. 4.Catalytic performance of PdCl2–CuCl2–KCl/AC catalysts with different preparation method. | |
Fig. 5 shows the catalytic performance of PdCl2–CuCl2–KCl/AC catalysts with different K promoter content, which were 0.3 wt%, 1.0 wt% and 2.0 wt%. From Fig. 5, it is noted that both STY of DMC and the selectivity of DMC based on MN and CO were higher over PdCl2–CuCl2–KCl/AC catalysts with different K promoter content than that of PdCl2–CuCl2/AC catalyst. The reactivity of PdCl2– CuCl2–KCl/AC catalysts increased when the mass content of K promoter increased from 0.3 wt% to 1.0 wt%. Meanwhile, when the mass content of K promoter increased from 1.0 wt% to 2.0 wt%, the catalytic activities of PdCl2–CuCl2–KCl/AC catalysts remained nearly unchanged. Therefore, 1.0 wt% was chosen as the optimal K promoter content of PdCl2–CuCl2–KCl/AC catalysts for DMC synthesis.

|
Download:
|
| Fig. 5.Catalytic performance of PdCl2–CuCl2–KCl/AC catalysts with different K promoter content. | |
The STY of DMC and selectivity of DMC based on MN and CO over PdCl2–CuCl2/AC catalysts with different K promoters ranked in the following order: PdCl2–CuCl2–KCl/AC > PdCl2–CuCl2/ AC > PdCl2–CuCl2–KOH/AC > PdCl2–CuCl2–CH3COOK/AC > PdCl2 –CuCl2–K2CO3/AC. Especially, the addition of KCl significantly improved the catalytic activities of PdCl2–CuCl2/AC catalyst for DMC synthesis from CO and MN. PdCl2–CuCl2/AC catalysts with different K promoters were characterized by N2 adsorption,H2-TPR and XPS techniques. The N2 adsorption data showed that the textural properties of all the catalysts were similar, while the H2- TPR results proved that the reduction temperature of Cu2+ species for all the catalysts ranked in the order: PdCl2–CuCl2–KCl/ AC < PdCl2–CuCl2–KOH/AC < PdCl2–CuCl2–CH3COOK/AC < PdCl2 –CuCl2–K2CO3/AC. The amount of Cu2+ in the PdCl2–CuCl2–KCl/AC catalyst was higher than that in the PdCl2–CuCl2/AC catalyst which was evidenced by the XPS results. The existence of KCl promoted the reducibility of Cu2+ species and increased the proportion of Cu2+ species on PdCl2–CuCl2–KCl/AC catalyst, which further benefited the redox cycle between Pd2+/Pd0 and Cu+/Cu2+ pairs and resulted in the better catalytic performance of PdCl2–CuCl2– KCl/AC catalyst for DMC synthesis from CO and MN.
AcknowledgmentsFinancial support from the Natural Science Foundation of China (Nos. 20936003, 21325626, 21176179), the Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP) (No. 20090032110021) is gratefully acknowledged.
| [1] | Y. Ono, Dimethyl carbonate for environmentally benign reactions, Catal. Today 35 (1997) 15-25. |
| [2] | M.A. Pacheco, C.L. Marshall, Review of dimethyl carbonate (DMC) manufacture and its characteristics as a fuel additive, Energy Fuels 11 (1997) 2-29. |
| [3] | P. Tundo, M. Selva, The chemistry of dimethyl carbonate, Acc. Chem. Res. 35 (2002) 706-716. |
| [4] | B. Ferrer, M. Alvaro, H. Garcia, Application of dimethyl carbonate as solvent and reagent, in: A. Mohammad (Ed.), Green Solvents I, Springer, Netherlands, 2012, pp. 363-374. . |
| [5] | C.S. Ló pez-Garzó n, L.A.M. van der Wielen, A.J.J. Straathof, Green upgrading of succinate using dimethyl carbonate for a better integration with fermentative production, Chem. Eng. J. 235 (2014) 52-60. . |
| [6] | Y.-J. Zhou, M. Xiao, S.-J. Wang, et al., Effects of Mo promoters on the Cu-Fe bimetal catalysts for the DMC formation from CO2 and methanol, Chin. Chem. Lett.24 (2013) 307-310. |
| [7] | J. Bian, X.W. Wei, L. Wang, Z.P. Guan, Graphene nanosheet as support of catalytically active metal particles in DMC synthesis, Chin. Chem. Lett. 22 (2011) 57-60. |
| [8] | T. Matsuzaki, A. Nakamura, Dimethyl carbonate synthesis and other oxidative reactions using alkyl nitrites, Catal. Surv. Asia 1 (1997) 77-88. |
| [9] | Y. Yamamoto, T. Matsuzaki, S. Tanaka, et al., Catalysis and characterization of Pd/ NaY for dimethyl carbonate synthesis from methyl nitrite and CO, J. Chem. Soc., Faraday Trans. 93 (1997) 3721-3727. |
| [10] | T. Matsuzaki, K. Ohdan, M. Asano, et al. Preparation method of dimethyl carbonate using methyl nitrite, Nippon Kagaku Kaishi 1999 (1999) 15-24. |
| [11] | Y. Yamamoto, Vapor phase carbonylation reactions using methyl nitrite over Pd catalysts, Catal. Surv. Asia 14 (2010) 103-110. |
| [12] | T. Matsuzaki, 99 Novel method for dimethyl carbonate synthesis using methyl nitrite, in: M. Anpo, M. Onaka, H. Yamashita (Eds.), Studies in Surface Science and Catalysis, Elsevier, 2003, pp. 447-450.. |
| [13] | P. Yang, Y. Cao, W.-L. Dai, J.-F. Deng, K.-N. Fan, Effect of chemical treatment of activated carbon as a support for promoted dimethyl carbonate synthesis by vapor phase oxidative carbonylation of methanol over Wacker-type catalysts, Appl. Catal. A: General 243 (2003) 323-331. |
| [14] | R.X. Jiang, S.F. Wang, X.Q. Zhao, Y.J. Wang, C.F. Zhang, The effects of promoters on catalytic properties and deactivation-regeneration of the catalyst in the synthesis of dimethyl carbonate, Appl. Catal. A: General 238 (2003) 131-139. |
| [15] | X.S. Ding, X.M. Dong, D.T. Kuang, et al., Highly efficient catalyst PdCl2-CuCl2-KOAc/ AC@Al2O3 for gas-phase oxidative carbonylation of methanol to dimethyl carbonate: preparation and reaction mechanism, Chem. Eng. J. 240 (2014) 221-227. |
| [16] | Y. Cao, P. Yang, C.-Z. Yao, et al., Impact of preparation strategy on the properties of carbon-supported Wacker-type catalysts in vapor-phase dimethyl carbonate synthesis, Appl. Catal. A: General 272 (2004) 15-22. |
| [17] | K. Tomishige, T. Sakaihori, S.-I. Sakai, K. Fujimoto, Dimethyl carbonate synthesis by oxidative carbonylation on activated carbon supported CuCl2 catalysts: catalytic properties and structural change, Appl. Catal. A: General 181 (1999) 95-102. |
| [18] | L. Wang, Y.B. Zhou, Q.F. Liu, Y. Guo, G.Z. Lu, Effect of surface properties of activated carbon on CO oxidation over supported Wacker-type catalysts, Catal. Today 153 (2010) 184-188. |
| [19] | A. Punnoose, M.S. Seehra, B.C. Dunn, E.M. Eyring, Characterization of CuCl2/PdCl2/ activated carbon catalysts for the synthesis of diethyl carbonate, Energy Fuels 16 (2001) 182-188. |
| [20] | L. Wang, Y.F. Feng, Y.H. Zhang, et al., Effect of original activated carbon support and the presence of NOx on CO oxidation over supported Wacker-type catalysts, Fuel 96 (2012) 440-445. |





