Heterocyclic compounds widely exist in the nature with widespread biological activities and they also have been assigned as privileged structures in drug development. For example, 2,3- dihydroquinazolin-4(1H)-ones are an important class of heterocycles with a broad spectrum of biological and pharmaceutical activities, such as anticancer, analgesic, antitumor and diuretic. Some 2,3-dihydro-4(1H)-quinazolinones are herbicides and plant growth regulators [1, 2]. Furthermore, they could be oxidized to 2- substituted-4(1H)-quinazolinones [3], which exhibit many central nervous system effects, cardiovascular and anti-inflammatory activity, and act as psychotropic, hypnotic, cardiotonic and antihistamine agents. Quinazolinones are potent antibacterial, antifungal, antiviral, antimycobacterial and antimalarial substances. They are also used as inhibitors of various enzymes including monoamine oxidase, aldose reductase, tumor necrosis factor R and thymidylate synthase [4].
As a result, anumber of synthetic strategies have been developed for the preparation of 2,3-dihydroquinazolin-4(1H)-ones or quinazolin- 4(3H)-ones. The most common approach involves condensation of 2-anthranilamide with structurally diverse aldehydes or ketones in the presence of various catalysts, such as 2-morpholinoethanesulfonic acid [5], p-TSA/NaHSO3[6], TiCl4/Zn [7], CuCl2[8], ionic liquid water [9], TFA [10], ammonium chloride [11], chiral phosphoric acids [12], HCl [13] and SmI2 [14]. Many of the reported synthetic protocols suffered from unsatisfactory product yields, critical product isolation procedures, volatile organic solvents and harsh reaction conditions such as high reaction temperatures, strong acidic media and prolonged reaction time.
In addition, there is a paucity of synthetic route to implement the selectivity of two such compounds [15]. Many of the reported methods are associated with the use of extra-oxidant to complete the fusion of the quinazolin-4(3H)-ones. Traditionally, at least a stoichiometricamountof toxic oxidants, such asDDQ[16], CuCl2 [17], MnO2 [18], KMnO4 [19], K2(S2O8) [20] and PhI(OAc)2[21]were used with possible uncontrollable explosive danger yielding almost the same amount of oxidant-derived waste. On the other hand, aerobic oxidationis interesting in keepingwiththe notion of green chemistry, sincemolecular oxygen is a cleaner and larger atom-efficient oxidant than other oxidants. Furthermore, molecular oxygen theoretically produces only water as the by-product after oxidation.
Herein, we report a versatile procedure for the fabrication of quinazolin-4(3H)-ones and 2,3-dihydroquinazolin-4(1H)-ones selectively catalyzed by Y(OTf)3 (Scheme 1). This methodology utilizes oxygen in air as oxidant in terms of green chemistry and atom economy and two target compounds were obtained by employing different solvent. We also demonstrate that yttrium is mild catalyst to efficiently activate the oxygen.

|
Download:
|
| Scheme. 1.The process to dihydroquinazolinone and quinazolinone derivatives. | |
1H NMR and 13C NMR spectra were made on Bruker ARX 400 for proton and carbon using DMSO-d6 or CDCl3 as the solvent with tetramethylsilane as an internal standard at room temperature. All reagents were obtained from commercial sources and used as received.
A general procedure for preparation of dihydroquinazolinones 3: Y(OTf)3 (0.0268 g, 0.05 mmol) were dissolved in 10 mL EtOH and stirred until the solid dissolved completely, then anthranilamide 1a (0.1498 g, 1.1 mmol) and benzaldehyde 2a (0.101 mL, 1.0 mmol) was added into the reaction mixture. After 1.5 h, the reaction was completed which was determined by TLC analysis. Water (15 mL) was added to the reaction mixture, and the crystalline products were collected by filtration to give the crude product. The crude products thus obtained were crystallized from EtOH to give pure products 3a.
A general procedure for preparation of quinazolinones 4: Y(OTf)3 (0.0268 g, 0.05 mmol) were dissolved in 10 mL DMSO and stirred until the solid dissolved completely, then anthranilamide 1a (0.1498 g, 1.1 mmol) and benzaldehyde 2a (0.101 mL, 1.0 mmol) was added into the reaction mixture. Then the mixture was heated to 110 °C under air atmosphere. After completion of the reaction monitored by TLC, the reaction mixture was cooled to room temperature. Water (15 mL) was added to the reaction mixture, and the crystalline products were collected by filtration to give the crude product. The crude products thus obtained were crystallized from EtOH to give pure products 4a. All 1H NMR and 13C NMR results were summarized in Supporting information.
3. Results and discussionInitially, we examined the model cyclocondensation reaction between anthanilamide (1) and benzaldehyde (2a) at 80°C in alcohol with altered catalysts, and the results are summarized in Table 1. It turned out that in the absence of the catalyst only trace product was obtained for even longer time (Table 1, entry 1). In the presence of Lewis catalysts, such as ZnCl2, CuCl2, FeCl3, the yields were dramatically improved (Table 1, entries 2–4). Interestingly, the reaction appears to work more efficiently using rare earth chlorides (Table 1, entries 5–9). Among the eight selected catalysts, Y(OTf)3 has proved to be the most effective (Table 1, entry 8).
| Table 1 Catalyst-screen for the synthesis of 3a.a |
To determine the effect of solvents, next we examined our reaction in different solvents as depicted in Table 2. Among all the solvents screened, it was found that alcohol was the most suitable solvent for this reaction (Table 2, entry 3). Interestingly, the reaction also proceeds in good yield at room temperature (Table 2, entry 4), which was chosen as the optimal condition for further work.
| Table 2 Screening of solvent for the synthesis of 3a.a |
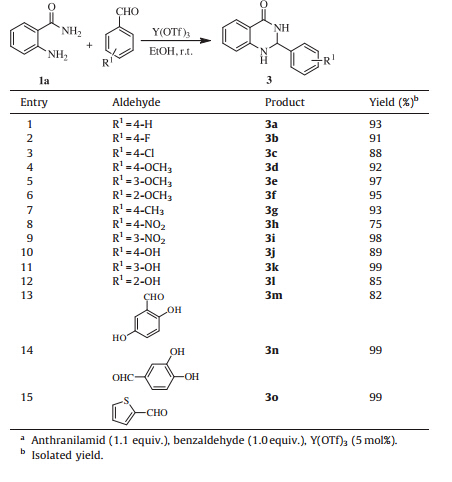
Encouraged by the above experiments, we further explored the synthetic protocol and the scope by employing different kinds of aldehydes functionalized with electron-rich and electron-deficient groups to form a series of dihydroquinazolinones. The results were summarized in Table 3. Clearly, all reactions worked well irrespective of substiuents on the aldehydes substrates (Table 3, entries 1–14). Heteroaromatic aldehyde was also readily introduced into the quinazolinone skeleton at 2-position, and desired product was formed in excellent yield (Table 3, entry 15).
| Table 3 One-pot synthesis of dihydroquinazolinones catalyzed by Y(OTf)3.a |
During the above reaction, trace of dihydroquinazolinones could be transformed to quinazolinones. The unexpected results prompted us to focus on the synthesis of quinazolin-4(3H)-one 4 using different oxidant such as H2O2, AATEMPOBF4-, NHPI, PhI(OAc)2, but with relatively unsatisfactory yield. To further improve the yield, this reaction proceeded in DMSO with the presence of Y(OTf)3. As expected, 4a was obtained in good yield while a trace amount of 3a was generated.
Then various aldehydes with either electron-donating or electron-withdrawing groups on aromatic ring were investigated in DMSO, and the results were listed in Table 4. It is noteworthy that various aldehydes were observed to be well tolerated under optimized conditions furnishing the product in good yields. Moreover, the reaction with nonaromatic aldehyde (Table 4, entry 18) also afforded the product in excellent yield.
| Table 4 One-pot synthesis of quinazolinones catalyzed by Y(OTf)3.a |
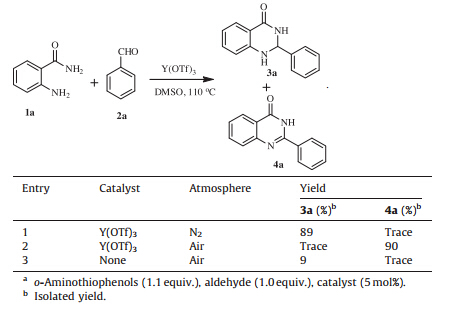
Then the roles of air and catalyst in the reaction were explored. Under nitrogen atmosphere, in the presence of Y(OTf)3, only a trace amount of quinazolinones 4a was detected whereas the dihydroquinalzolinone 3a was obtained in good yield (Table 5, entry 1). When the same reaction was carried out in air, the yield of 4a reached nearly 100% (Table 5, entry 2). It can therefore be deduced that oxygen in air as an oxidant promotes the transformation of 3a to product 4a. In the absence of catalyst, compound 3a was produced only in 9% yield while 4a in trace yield in air, indicating that the catalyst plays an important role not only in the condensation process but also the oxidation.
| Table 5 Effect of atmosphere and catalyst in the formation of 3a and 4a.a |

|
Download:
|
| Scheme. 2.Proposed reaction mechanism for the synthesis of 3a and 4a. | |
In conclusion, we have developed a green and efficient method to selectively synthesize dihydroquinazolinones and quinazolinones catalyzed by Y(OTf)3 via regulating solvents. This method provides a simple, compatible and potentially powerful synthetic protocol for the modular construction of quinazolinones and dihydroquinazolinones, which are important units of biologically active molecules. The usage of molecular oxygen in air renders this protocol very attractive and practical. Furthermore, our results demonstrated that the rare earth catalysts play a decisive role in aerobic oxidation. A detailed study on the scope of our synthetic methodology is ongoing in our laboratory.
AcknowledgmentWe gratefully acknowledge the National Natural Science Foundation of China (No. 20802052) for financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015.07. 026.
| [1] | (a) M. Singh, N. Raghav, 2,3-Dihydroquinazolin-4(1H)-one derivatives as potential non-peptidyl inhibitors of cathepsins B and H, Bioorg. Chem. 59 (2015) 12-22; (b) X.F. Wu, S. Oschatz, A. Block, A. Spannenberg, P. Langer, Base mediated synthesis of 2-aryl-2,3-dihydroquinazolin-4(1H)-ones from 2-aminobenzonitriles and aromatic aldehydes in water, Org. Biomol. Chem. 12 (2014) 1865-1870; (c) A.J. Watson, A.C. Maxwell, J.M. Williams, Ruthenium-catalysed oxidative synthesis of heterocycles from alcohols, Org. Biomol. Chem. 10 (2012) 240-243. |
| [2] | (a) M. Yamato, J. Horiuchi, Y. Takeuchi, Reaction of 1,2,3,4-tetrahydroquinazolin- 4-ones with acid anhydride. III, Chem. Pharm. Bull. 29 (1981) 3124-3129; (b) E. Hamel, C.M. Lin, J. Plowman, et al., Antitumor 2,3-dihydro-2-(aryl)-4(1H)- quinazolinone derivatives, Biochem. Pharmacol. 51 (1996) 53-59. |
| [3] | M. Bakavoli, O. Sabzevari, M. Rahimizadeh, Microwave activated synthesis of 2- aryl-quinazolin-4(3H)ones, Chin. Chem. Lett. 18 (2007) 1466-1468. |
| [4] | (a) K.A. Stephenson, A.A. Wilson, S. Houle, N. Vasdev, Synthesis and in vitro evaluation of derivatives of the beta(1)-adrenergic receptor antagonist HX-CH 44, Bioorg. Med. Chem. Lett. 21 (2011) 5506-5509; (b) R. Potter, A.G. Horti, H.T. Ravert, et al., Synthesis and in vivo evaluation of (S)- 6-(4-fluorophenoxy)-3-((1-[11C]methylpiperidin-3-yl)methyl)-2-o-tolylquinazol in-4(3H)-one, a potential PET tracer for growth hormone secretagogue receptor (GHSR), Bioorg. Med. Chem. 19 (2011) 2368-2372; (c) S.E. Napier, J.J. Letourneau, N. Ansari, et al., Synthesis and SAR studies of novel 2-(6-aminomethylaryl-2-aryl-4-oxo-quinazolin-3(4H)-yl)acetamide vasopressin V1b receptor antagonists, Bioorg. Med. Chem. Lett. 21 (2011) 3813-3817; (d) E. Manivannan, S.C. Chaturvedi, Analogue-based design, synthesis and molecular docking analysis of 2,3-diaryl quinazolinones as non-ulcerogenic antiinflammatory agents, Bioorg, Med. Chem. 19 (2011) 4520-4528. |
| [5] | V.B. Labade, P.V. Shinde, M.S. Shingare, A facile and rapid access towards the synthesis of 2,3-dihydroquinazolin-4(1H)-ones, Tetrahedron Lett. 54 (2013) 5778-5780. |
| [6] | M.J. Hour, L.J. Huang, S.C. Kuo, et al., 6-Alkylamino- and 2,3-dihydro-3'-methoxy- 2-phenyl-4-quinazolinones and related compounds: their synthesis, cytotoxicity, and inhibition of tubulin polymerization, J. Med. Chem. 43 (2000) 4479-4487. |
| [7] | D. Shi, L. Rong, J. Wang, et al., Synthesis of quinazolin-4(3H)-ones and 1,2- dihydroquinazolin-4(3H)-ones with the aid of a low-valent titanium reagent, Tetrahedron Lett. 44 (2003) 3199-3201. |
| [8] | R.J. Abdel-Jalil, W. Voelter, M. Saeed, A novel method for the synthesis of 4(3H)- quinazolinones, Tetrahedron Lett. 45 (2004) 3475-3476. |
| [9] | J. Chen, W. Su, H. Wu, M. Liu, C. Jin, Eco-friendly synthesis of 2,3-dihydroquinazolin- 4(1H)-ones in ionic liquids or ionic liquid-water without additional catalyst, Green Chem. 9 (2007) 972-975. |
| [10] | G.M. Chinigo, M. Paige, S. Grindrod, et al., Asymmetric synthesis of 2,3-dihydro-2- arylquinazolin-4-ones: methodology and application to a potent fluorescent tubulin inhibitor with anticancer activity, J. Med. Chem. 51 (2008) 4620-4631. |
| [11] | A. Shaabani, A. Maleki, H. Mofakham, Click reaction: highly efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones, Synth. Commun. 38 (2008) 3751-3759. |
| [12] | (a) X. Cheng, S. Vellalath, R. Goddard, B. List, Direct catalytic asymmetric synthesis of cyclic aminals from aldehydes, J. Am. Chem. Soc. 130 (2008) 15786- 15787; (b) M. Rueping, A.P. Antonchick, E. Sugiono, K. Grenader, Asymmetric Bronsted acid catalysis: catalytic enantioselective synthesis of highly biologically active dihydroquinazolinones, Angew. Engl.Chem. Int. Ed. 48 (2009) 908-910. |
| [13] | L.H. Klemm, T.J.R. Weakley, R.D. Gilbertson, Y.H. Song, Definitive structural assignment of condensation products from anthranilamide and 3-amino-2-carbamoylthiophene with ketones. Formation of tetrahydroquinazolinones and their thiophene isosteres, J. Heterocycl. Chem. 35 (1998) 1269-1273. |
| [14] | G. Cai, X. Xu, Z. Li, P. Lu, W.P. Weber, A one-pot synthesis of 2-aryl-2,3-dihydro- 4(lH)-quinazolinones by use of samarium iodide, J. Heterocycl. Chem. 39 (2002) 1271-1272. |
| [15] | L.Y. Zeng, C. Cai, Iodine: selectively promote the synthesis of mono substituted quinazolin-4(3H)-ones and 2,3-dihydroquinazolin-4(1H)-ones in one-pot, J. Heterocycl. Chem. 47 (2010) 1035-1039. |
| [16] | Y. Mitobe, S. Ito, T. Mizutani, et al., Development of a selective and potent radioactive ligand for histamine H(3) receptors: a compound potentially useful for receptor occupancy studies, Bioorg. Med. Chem. Lett.19 (2009) 4075-4078. |
| [17] | W. Voelter, R.J. Abdel-Jalil, H.M. Aldoqum, M.T. Ayoub, Synthesis and antitumor activity of 2-aryl-7-fluoro-6-(4-methyl-1-piperazinyl)-4(3H)-quinazolinones, Heterocycles 65 (2005) 2061-2070. |
| [18] | C. Balakumar, P. Lamba, D.P. Kishore, et al., Synthesis, anti-inflammatory evaluation and docking studies of some new fluorinated fused quinazolines, Eur. J. Med. Chem. 45 (2010) 4904-4913. |
| [19] | Y. Zheng, M. Bian, X.Q. Deng, S.B. Wang, Z.S. Quan, Synthesis and anticonvulsant activity evaluation of 5-phenyl-[1,2,4]triazolo[4,3-c]quinazolin-3-amines, Arch. Pharm. (Weinheim) 346 (2013) 119-126. |
| [20] | A. Kumar, R.A. Maurya, D. Saxena, Diversity-oriented synthesis of benzimidazole, benzoxazole, benzothiazole and quinazolin-4(3H)-one libraries via potassium persulfate-CuSO4-mediated oxidative coupling reactions of aldehydes in aqueous micelles, Mol. Divers 14 (2010) 331-341. |
| [21] | K. Zhao, Y. Du, R. Cheng, T. Guo, D. Zhang-Negrerie, One-Pot synthesis of quinazolinones from anthranilamides and aldehydes via p-toluenesulfonic acid catalyzed cyclocondensation and phenyliodine diacetate mediated oxidative dehydrogenation, Synthesis 45 (2013) 2998-3006. |