b Suzhou Institute of Wuhan University, Suzhou 215123, China
Hydrogen is considered as one of the most important clean fuel for the sustainable development because of its merit of high efficiency and power density [1]. Safe and controlled storage of hydrogen are the widely known barriers in the fuel cell based hydrogen economy [2]. Recently,ammonia borane (NH3-BH3,AB) has been regarded as one of the best potential chemical hydrogen storage candidate because of its 19.6 wt% hydrogen content,highly stability,and environmental benignity [3]. Meanwhile,methylamine borane (CH3NH2-BH3,MeAB),the derivative of AB has also been studied due to its high gravimetric hydrogen content (11.1 wt%). The hydrogen release from hydrolysis of AB/MeAB could be as much as 3 mol of hydrogen per mol of AB/MeAB with an appropriate catalyst according to Eqs. (1) and (2) [4],which seems to be the most convenient way for portable hydrogen storage applications compared with solid phase thermolysis [5],and catalytic dehydrogenation in non-aqueous solvents [6].

So far,a lot of metal nanoparticles (NPs) based catalysts have been developed for catalytic hydrolysis of AB and MeAB [7, 8, 9],among them,the Ru based nanocatalysts have been identified as one of themost effective catalysts. However,their catalytic activities are highly depended on the dispersion of active metal sites [10]. In order to avoid the aggregation,many methods have been employed in their synthesis route. For example,ö zkar reported laurate stabilized RuNPswith TOF of 75 min-1 [11],Miele reported γ-Al2O3 supported Ru NPs with turnover frequency (TOF) value of 77 min-1 toward catalytic hydrolysis of AB [12],and multiwalled carbon nanotube supported Ru NPs with TOF of 329min-1 [13],Ma reported carbon black supported Ru NPs with TOF of 429 min-1 [14]. Very recently,our group reported microporousmetal-organic frameworks MIL-96 supported Ru NPs with TOF of 231 min-1 [15],and MIL-101 immobilized Ru NPs with TOF of 178 min-1 [16]. As a continuous work to study the pore size effect to the effect of catalytic activity of Ru NPs,MCM-41 a mesoporous material with high special surface areas,large pore volumes,andhomogeneous pore arrays [17],which consists of ordered hexagonal pores has been used as supported material for Ru NPs. Herein,Ru NPs have been firstly deposited on MCM-41,and further tested for catalytic hydrolysis of AB andMeAB.
2. Experimental 2.1. Chemicals and materialsAll chemicals were commercial and used without further purification. MCM-41 (Sinopharm Chemical Reagent Co.,Ltd.),ruthenium chloride hydrate (RuCl3.nH2O,Wuhan Greatwall Chemical Co.,Ltd.,99%),ammonia borane (NH3BH3,AB,United Boron (Zhengzhou) Energy Materials S&T LLC.,98%),methylamine hydrochloride (CH3NH2.HCl,Sinopharm Chemical Reagent Co.,Ltd.,≥96%),sodium borohydride (NaBH4,Sinopharm Chemical Reagent Co.,Ltd.,96%),tetrahydrofuran (C4H8O,Sinopharm Chemical Reagent Co.,Ltd.,≥99%),diethyl ether anhydrous (C4H10O,Sinopharm Chemical Reagent Co.,Ltd.,≥99.7%),ethanol (C2H5OH,Sinopharm Chemical Reagent Co.,Ltd.,>99.8%),ketjen black EC-300J (Triquo Chemical Co.,Ltd.),neutral silica power (SiO2,Branch of Qingdao Haiyang Chemical Co.,Ltd.),were used as received. We use ordinary distilled water as the reaction solvent.
2.2. Synthesis of methyl ammonia borane (CH3NH2-BH3,MeAB)MeAB was synthesized by the method reported in the literature [18]. Methylamine hydrochloride and sodium borohydride were added to a flask. The mixure of contents were vigorously stirred when THF was transferred into the flask. The resultant solution was filtered by suction filtration after the reaction was carried out for 12 h,and the filtrate was concentrated. MeAB was obtained from purification by diethyl ether.
2.3. Synthesis of Ru/MCM-41MCM-41 (100 mg) was mixed with 0.01,0.02,0.03,0.04 mmol RuCl3 solution (0.01 mol L-1) in a two-necked round-bottom flask containing 10 mL deionized water for 24 h at room temperature. The resulting mixture was then reduced with AB (30.8 mg,1 mmol) at 293 K to yield Ru/MCM-41.
2.4. Hydrolysis of AB by Ru/MCM-4150 mg Ru/MCM-41 with different loadings was kept in a twonecked round-bottom flask containing 10 mL de-ionized water. A pressure-equalization funnel was connected to one neck to introduce aqueous solution of AB (30.8 mg,1 mmol) and a gas buret was connected to the other neck to record the volume of the gas evolution. As soon as the aqueous solution was added to the catalyst,the volume of the gas evolution was monitored immediately. All the reactions were carried out at 298 K in air.
2.5. Hydrolysis of MeAB by Ru/MCM-4150 mg Ru/MCM-41 was kept in a two-necked round-bottom flask containing 10 mL de-ionized water. A pressure-equalization funnel was connected to one neck to introduce aqueous solution of MeAB (45 mg,1 mmol),and the other neck was to record the volume of the gas evolution. As soon as the aqueous solution was added to the catalyst,the volume of the gas evolution was monitored immediately. All the reactions were carried out at 298 K in air.
2.6. In situ synthesis of Ru supported on different materials and their application toward hydrolysis of ammonia borane50 mg different supported materials (carbon black,SiO2) were mixed separately with 0.0055 mmol RuCl3 solution (0.01 mol L-1) and kept in a two-necked round-bottom flask containing 10 mL deionized water. After aqueous solution of AB (30.8 mg,1 mmol) was introduced,Ru3+ was reduced to Ru0. Then the new fresh aqueous solution of AB (30.8 mg,1 mmol) was added when the hydrogen generation reaction was completed. All the reactions were carried out at 298 K in air.
2.7. Hydrolysis of AB catalyzed by MCM-4150 mg Ru/MCM-41 was substituted by 50 mg MCM-41,the experiment procedures were similar to that 2.4 procedure. In order to determine the rate law of the catalytic hydrolysis of the AB or MeAB,the concentration was kept unchanged (1 mmol). Also the reactions were carried out at 25 8C,30 8C,35 8C and 40 8C,while 50 mg Ru/MCM-41 and AB or MeAB (1 mmol) were kept the same to obtain the activation energy (Ea).
2.8. Cycle stability testAB or MeAB (1 mmol) solution was introduced to 5 mL of water dispersed 50 mg Ru/MCM-41,the evolution of gas was recorded as described above. As soon as the hydrogen generation reaction was completed,another new fresh equivalent of AB or MeAB (1 mmol) was added to the mixure. Such cycle stability tests of the catalyst were carried out five times at 298 K in air.
2.9. CharacterizationThe morphologies and sizes of different samples were characterized by using a Tecnai G20 U-Twin transmission electron microscope (TEM) equipped with an energy dispersive X-ray detector (EDX) at an acceleration voltage of 200 kV. The surface area measurements were performed with N2 adsorption/desorption isotherms at liquid nitrogen temperature (77 K) after dehydration under vacuum at 150 8C for 18 h using Quantachrome NOVA 4200e. The inductively coupled plasma-atomic emission spectroscopy (ICP-AES) was performed on IRIS Intrepid II XSP (Thermo Fisher Scientific,USA). Powder X-ray diffraction (XRD) patterns were measured by a Bruker D8-Advance X-ray diffractometer using Cu Ka radiation source (λ = 0.154178 nm) with a velocity of 18 min-1.
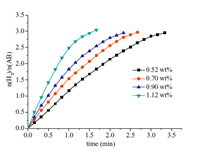
3. Results and discussionThe MCM-41 supported Ru catalyst was synthesized through a simple liquid impregnation-reduction method. The loading values of Ru deposited on MCM-41 of different samples were determined as 0.52,0.70,0.90 and 1.12 wt% performed on the inductively coupled plasma atomic emission spectroscopic (ICP-AES). In order to verify the integrity and regularity of the mesoporous structure,MCM-41,Ru/MCM-41 characterized by the powder X-ray diffractions (PXRD) show no loss of crystallinity (Fig. 1),which corresponds to the reported MCM-41 zeolite [19],demonstrating that the integrity and regularity of MCM-41 structure maintained perfect and intact during the catalyst preparation and catalytic process. Furthermore,no obvious diffraction peaks of Ru were observed from the wide-angle PXRD (Fig. 1b),which might be caused by the traps of Ru NPs into the pores of MCM-41 and the ultrafine Ru NPs (vide infra). The adsorption-desorption isotherms of MCM-41 and Ru/MCM-41 were shown in Fig. 2a. The specific areas of MCM-41 and Ru/MCM-41 were 900 and 779 m2 g-1,respectively. The decrease in the amount of N2 adsorption and the pore volume (Fig. 2a,Table 1) of Ru/MCM-41 indicates that the pores of MCM-41 were either occupied by the well dispersed Ru NPs or blocked by the Ru NPs. The morphologies of MCM-41 supported Ru NPs were further characterized by transmission electron microscopic (TEM),energy-dispersive X-ray spectroscopy (EDX) measurements. TEM images of Ru/MCM-41 present small NPs appeared as dark spots which deposited on the mesoporous pores (Fig. 3a,b),indicating the Ru NPs are well dispersed with mean diameter of 2.6 nm (Fig. 3d). The steric restrictions from ordered hexagonal porous structures of MCM-41may restrain and confine the growth of Ru NPs on the surface,which could be accommodated in the mesoporous pores with diameters 3.4 nm (Fig. 2b). The EDX spectra further confirmed the presence of Ru (Fig. 3c). Fig. 4 shows the H2 generation from aqueous AB at ambient conditions in the present of Ru/MCM-41. Among the catalysts of Ru/MCM-41 with different Ru loadings (0.52,0.70,0.90 and 1.12 wt% determined by ICP-AES),1.12 wt% Ru/MCM-41 with the TOF value of 288 mol H2 min-1 (mol Ru)-1,this value is among the highest value ever reported (Table 2). Furthermore,the catalytic activity of Ru supported on different materials toward hydrolysis of AB was studied. As shown in Fig. 5,compared with other conventional supports,such as SiO2,carbon black,Ru/MCM- 41 exhibits the superior catalytic activity. Moreover,Ru NPs without supported materials and MCM-41 without Ru loading were synthesized and applied to hydrolysis of AB,only 2.6 equiv. H2 were released for more than 17 min for Ru NPs,and almost no reactivity for MCM-41. These results emphasis on the dominant factor in facilitating hydrolysis of AB is the synergistic effect between MCM-41 and Ru NPs. The intrinsic activity of the active sites,potential effects of adsorption and steric restrictions of pore confinement from MCM-41 may help to activate the reactants and promote the reaction.

|
Download:
|
| Fig. 1.(a) The small-angle PXRD patterns of samples. (b) The wide-angle PXRD patterns of samples. | |

|
Download:
|
| Fig. 2.(a) N2 sorption isotherms of MCM-41 and Ru/MCM-41. (b) The pore diameter distribution of MCM-41 and Ru/MCM-41. | |

|
Download:
|
| Fig. 3.(a, b) TEM images of Ru/MCM-41. (c) EDX of 1.12 wt% Ru/MCM-41. (d) Ru nanoparticle size distribution histogram of 1.12 wt% Ru/MCM-41, mean size = 2.6 nm. | |
|
Download:
|
| Fig. 4.Hydrogen generation from aqueous AB in the presence of Ru/MCM-41 catalysts at room temperature. Ru/AB (molar ratio) = 0.0026, 0.0044, 0.0045 and 0.0055 at Ru loadings of 0.52, 0.70, 0.90 and 1.12 wt%. | |

|
Download:
|
| Fig. 5.Hydrogen generation from the hydrolysis of AB catalyzed by Ru/MCM-41, Ru/ C black, Ru/SiO2, Ru and MCM-41 (Ru/AB = 0.0055). | |
| Table 1 Pore volume and surface area of MCM-41 and Ru/MCM-41. |
| Table 2 Catalytic activity of Ru-based and some noble metal catalysts used for hydrolytic dehydrogenation of AB. |
The activation energy (Ea) of the AB or MeAB hydrolysis catalyzed by Ru/MCM-41 were gotten by carrying out the hydrolytic reaction varied at 25 8C,30 8C,35 8C and 40 8C. The values of rate constant κ at different temperatures were obtained from the slope of the linear part of each plot from Figs. 6a and 8a. The Arrhenius plot of ln κ vs. 1/T for the catalyst is plotted in Figs. 6b and 8b,from which the apparent activation energy was determined to be approximately 28.03 kJ mol-1 and 47.60 kJ mol-1 for AB and MeAB,respectively. The cycle stability of the catalyst is important in the practical application. The cycle stability of Ru/MCM-41 catalyst for hydrolysis was invested by adding another new fresh equivalent of AB or MeAB after the last hydrogen generation reaction was complete. As shown in Figs. 7 and 9,the as-synthesized Ru/MCM-41 catalysts retain about 73% and 61% of their initial catalytic activity toward hydrolysis of AB and MeAB in the fifth run,respectively. The reason for slight decrease of the catalytic activity might be the increase viscosity of the mixture solution during the hydrolysis of AB and MeAB [19]. Further study on improvement the cycle stability of the present catalyst for the hydrolysis of AB or MeAB is still underway.

|
Download:
|
|
Fig. 6.(a) Time course plots for hydrogen generation by the decomposition of AB by Ru/MCM-41 at different temperatures. (b) Plot of ln |
|

|
Download:
|
| Fig. 7.(a) Cycle stability test for the hydrogen generation from aqueous AB in the presence of Ru/MCM-41 catalyst (Ru/AB molar ratio = 0.0055). (b) percentage of initial catalytic activity of Ru/MCM-41 in successive runs after the reuse for the hydrolysis of AB. | |

|
Download:
|
|
Fig. 8.(a) Time course plots for hydrogen generation by the decomposition of MeAB by Ru/MCM-41 at different temperatures. (b) Plot of ln |
|

|
Download:
|
| Fig. 9.(a) Cycle stability test for the hydrogen generation from aqueous MeAB in the presence of Ru/MCM-41 catalyst (Ru/MeAB molar ratio = 0.0055). (b) Percentage of initial catalytic activity of Ru/MCM-41 in successive runs after the reuse for the hydrolysis of MeAB. | |
In summary,ultrafine Ru NPs have been well deposited on MCM-41,which exhibit highly catalytic activity toward hydrolysis of AB and MeAB at ambient conditions. Thanks to the unique structure of MCM-41 with ordered hexagonal pores,and the strong synergistic effect,Ru NPs supported on MCM-41 shows excellent catalytic activity compared with other commercial supported materials toward catalytic dehydrogenation from ammonia borane,with the TOF value of 288 mol H2 min-1 (mol Ru)-1.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21201134),the Natural Science Foundation of Jiangsu Province (No. BK20130370),the Natural Science Foundation of Hubei Province (No. 2013CFB288),and Large-scale Instrument and Equipment Sharing Foundation of Wuhan University.
| [1] | Y.X. Wang, T.H. Chen, A high dispersed Pt0.35Pd0.35Co0.30/C as superior catalyst for methanol and formic acid electro-oxidation, Chin. Chem. Lett. 25 (2014) 907-911. |
| [2] | W.H. Yuan, L.L. Mao, L. Li, Novel SrCe0.75Zr0.20Tm0.05O3 d membrane for hydrogen separation, Chin. Chem. Lett. 21 (2010) 369-372. |
| [3] | F.H. Stephens, V. Pons, R.T. Baker, Ammonia-borane: the hydrogen source par excellence? Dalton Trans. 25 (2007) 2613-2626. |
| [4] | Y.W. Yang, Z.H. Lu, Y.J. Hu, et al., Facile in situ synthesis of copper nanoparticles supported on reduced graphene oxide for hydrolytic dehydrogenation of ammonia borane, RSC Adv. 4 (2014) 13749-13752. |
| [5] | Z.X. Yang, F.Y. Cheng, Z.L. Tao, J. Liang, J. Chen, Decreasing the thermal dehydrogenation temperature of methylamine borane (MeAB) by mixing with poly(- methyl acrylate) (PMA), Int. J. Hydrogen Energy 37 (2012) 7638-7644. |
| [6] | A. Staubitz, M.E. Sloan, A.P. Robertson, et al., Catalytic dehydrocoupling/dehydrogenation of N-methylamine-borane and ammonia-borane: synthesis and characterization of high molecular weight polyaminoboranes, J. Am. Chem. Soc. 132 (2010) 13332-13345. |
| [7] | J.F. Shen, L. Yang, K. Hu, W. Luo, G.Z. Cheng, Rh nanoparticles supported on graphene as efficient catalyst for hydrolytic dehydrogenation of amine boranes for chemical hydrogen storage, Int. J. Hydrogen Energy 40 (2015) 1062-1070. |
| [8] | L. Yang, J. Su, W. Luo, G.Z. Cheng, Strategic synthesis of graphene supported trimetallic Ag-based core-shell nanoparticles toward hydrolytic dehydrogenation of amine boranes, Int. J. Hydrogen Energy 39 (2014) 3360-3370. |
| [9] | W.Q. Feng, L. Yang, N. Cao, et al., In situ facile synthesis of bimetallic CoNi catalyst supported on graphene for hydrolytic dehydrogenation of amine borane, Int. J. Hydrogen Energy 39 (2014) 3371-3380. |
| [10] | N. Cao, J. Su, W. Luo, G.Z. Cheng, Ni-Pt nanoparticles supported on MIL-101 as highly efficient catalysts for hydrogen generation from aqueous alkaline solution of hydrazine for chemical hydrogen storage, Int. J. Hydrogen Energy 39 (2014) 9726-9734. |
| [11] | F. Durap, M. Zahmakıran, S.Ö zkar,Water soluble laurate-stabilized ruthenium (0) nanoclusters catalyst for hydrogen generation from the hydrolysis of ammonia- borane: high activity and long lifetime, Int. J. Hydrogen Energy 34 (2009) 7223-7230. |
| [12] | M. Chandra, Q. Xu, Room temperature hydrogen generation from aqueous ammonia- borane using noble metal nano-clusters as highly active catalysts, J. Power Sources 168 (2007) 135-142. |
| [13] | S. Akbayrak, S.Ö zkar, Ruthenium nanoparticles supported on multiwalled carbon nanotube as highly active catalyst for hydrogen generation from ammonia borane, ACS Appl. Mater. Interfaces 4 (2012) 6302-6310. |
| [14] | H.Y. Liang, G.Z. Chen, S. Desinan, et al., In situ facile synthesis of ruthenium nanocluster catalyst supported on carbon black for hydrogen generation from the hydrolysis of ammonia-borane, Int. J. Hydrogen Energy 37 (2012) 17921-17927. |
| [15] | L. Wen, J. Su, X.J. Wu, et al., Ruthenium supported on MIL-96: an efficient catalyst for hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage, Int. J. Hydrogen Energy 39 (2014) 17129-17135. |
| [16] | N. Cao, T. Liu, J. Su, et al., Ruthenium supported on MIL-101 as an efficient catalyst for hydrogen generation from hydrolysis of amine boranes, New J. Chem. 38 (2014) 4032-4035. |
| [17] | R.H. Huang, J. Liu, L.S. Li, et al., Fe/MCM-41 as a promising heterogeneous catalyst for ozonation of p-chlorobenzoic acid in aqueous solution, Chin. Chem. Lett. 22 (2011) 683-686. |
| [18] | Y.S. Du, N. Cao, L. Yang, et al., One-step synthesis of magnetically recyclable rGO supported Cu@Co core-shell nanoparticles: highly efficient catalysts for hydrolytic dehydrogenation of ammonia borane and methylamine borane, New J. Chem. 37 (2013) 3035-3042. |
| [19] | L. Yang, W. Luo, G.Z. Cheng, Graphene-supported Ag-based core-shell nanoparticles for hydrogen generation in hydrolysis of ammonia borane and methylamine borane, ACS Appl. Mater. Interfaces 5 (2013) 8231-8240. |
| [20] | M. Rakap, Hydrogen generation from hydrolysis of ammonia borane in the presence of highly efficient poly (N-vinyl-2-pyrrolidone)-protected platinumruthenium nanoparticles, Appl. Catal. A: Gen. 478 (2014) 15-20. |
| [21] | S. Akbayrak, S. Tanyıldızı, I. Morkan, S. Ö zkar, Ruthenium (0) nanoparticles supported on nanotitania as highly active and reusable catalyst in hydrogen generation from the hydrolysis of ammonia borane, Int. J. Hydrogen Energy 39 (2014) 9628-9637. |
| [22] | F. Durap, M. Zahmakıran, S. Ö zkar, Water soluble laurate-stabilized rhodium (0) nanoclusters catalyst with unprecedented catalytic lifetime in the hydrolytic dehydrogenation of ammonia-borane, Appl. Catal. A: Gen. 369 (2009) 53-59. |
| [23] | Ö . Metin, Ş . Ş ahin, S.Ö zkar, Water-soluble poly(4-styrenesulfonic acid-co-maleic acid) stabilized ruthenium (0) and palladium (0) nanoclusters as highly active catalysts in hydrogen generation from the hydrolysis of ammonia-borane, Int. J. Hydrogen Energy 34 (2009) 6304-6313. |
| [24] | N. Cao, W. Luo, G.Z. Cheng, One-step synthesis of graphene supported Ru nanoparticles as efficient catalysts for hydrolytic dehydrogenation of ammonia borane, Int. J. Hydrogen Energy 38 (2013) 11964-11972. |
| [25] | M. Zahmakıran, S. Ö zkar, Zeolite framework stabilized rhodium (0) nanoclusters catalyst for the hydrolysis of ammonia-borane in air: outstanding catalytic activity, reusability and lifetime, Appl. Catal. B: Environ. 89 (2009) 104-110. |
| [26] | H. Can, Ö . Metin, A facile synthesis of nearly monodisperse ruthenium nanoparticles and their catalysis in the hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage, Appl. Catal. B: Environ. 125 (2012) 304-310. |
| [27] | Q. Xu, M. Chandra, A portable hydrogen generation system: catalytic hydrolysis of ammonia-borane, J. Alloys Compd. 446-447 (2007) 729-732. |
| [28] | H.M. Dai, J. Su, K. Hu, W. Luo, G.Z. Cheng, Pd nanoparticles supported on MIL-101 as high-performance catalysts for catalytic hydrolysis of ammonia borane, Int. J. Hydrogen Energy 39 (2014) 4947-4953. |
| [29] | N. Cao, J. Su, W. Luo, et al., Graphene supported Ru@Co core-shell nanoparticles as efficient catalysts for hydrogen generation from hydrolysis of ammonia borane and methylamine borane, Catal. Commun. 43 (2014) 47-51. |
| [30] | N. Cao, J. Su, W. Luo, et al., Hydrolytic dehydrogenation of ammonia borane and methylamine borane catalyzed by graphene supported Ru@Ni core-shell nanoparticles, Int. J. Hydrogen Energy 39 (2014) 426-435. |
| [31] | G.Z. Chen, S. Deinasn, R. Nechache, et al., Bifunctional catalytic/magnetic Ni@Ru core-shell nanoparticles, Chem. Commun. 47 (2011) 6308-6310. |
| [32] | N. Cao, J. Su, X.L. Hong, et al., In situ facile synthesis of Ru-based core-shell nanoparticles supported on carbon black and their high catalytic activity in the dehydrogenation of amine-boranes, Chem. Asian J. 9 (2014) 562-571. |
| [33] | P.X. Xi, F.J. Chen, G.Q. Xie, et al., Surfactant free RGO/Pd nanocomposites as highly active heterogeneous catalysts for the hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage, Nanoscale 4 (2012) 5597-5601. |
| [34] | G.P. Rachiero, U.B. Demirci, P. Miele, Bimetallic RuCo and RuCu catalysts supported on g-Al2O3. A comparative study of their activity in hydrolysis of ammonia- borane, Int. J. Hydrogen Energy 36 (2011) 7051-7065. |
| [35] | N. Cao, K. Hu, W. Luo, et al., Ru/Cu nanoparticles supported on graphene: a highly efficient catalyst for hydrolysis of ammonia borane, J. Alloys Compd. 590 (2014) 241-246. |
 2015, Vol.26
2015, Vol.26 




