b Emergency Department, China-Japan Union Hospital of Jilin University, Changchun 130033, China
Interest in the development of dissolved oxygen sensing devices is very extensive in the environmental,medical and industrial fields [1]. When compared to other techniques employed for oxygen determination,such as optical methods, electrochemical techniques have the advantages of simplicity and high sensitivity. The oxygen reduction reaction (ORR) is the key point in the process,where the amount of dissolved oxygen can improve or inhibit the process [2]. In order to promote the development and wide application of a sensor,studies to identify (or determine) an efficient,cheap,stable,and green non-precious metal ORR catalyst have been a top priority [3, 4, 5]. Hence,batch syntheses of well-controlled and low-cost carbon composite ORR catalysts became one of the greatest current challenges in the field of preparation of non-precious metal ORR catalyst [6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18]. To date, there exists tremendous preoccupation in exploring a simple method to prepare stable carbon composites from renewable biomass resources including soy milk,wood,bamboo,fruits,kenaf stem,egg white,and bacteria for potential applications in super capacitors,energy storage,electroanalytical chemistry,and electrocatalysts [19, 20, 21, 22, 23].
The Jia group had prepare a porous carbon composite membrane (PCCM) from low-cost common filter paper (FP) which acts as the carbon precursor [24, 25, 26]. The Fe-based nanoparticles formed on the composite surface during the pyrolysis may further improve the electrocatalytic ORR activity of the PCCM,and the porous architecture of this FP-derived composite is beneficial to O2 adsorption and transport during the ORR process. In order to investigate the characteristics of the PCCM,we fabricated the PCCM on the surface of the GCE.The electrochemical properties of the PCCM coated glassy carbon electrode for reducing dissolved oxygen in an alkaline environment were investigated. In this context,the present work reports on the development of an efficient and stable sensor for oxygen determination based on the construction of a novel PCCM produced from low-cost common FP, which acts as the carbon precursor on the surface of GCE.
The resulting simple,cost-effective,sensitive,and selective dissolved oxygen sensor exhibits fast response (10 s to reach steady state) and a low detection limit. Optimization of the experimental conditions yielded a detection limit and sensitivity for O2 much better than those described in the literature. This sensor showed good repeatability for both the electrode preparation and determinations,evaluated in term of relative standard deviations,as well as allied with a simple and easy method of preparation. In view of these intrinsic advantages,this novel type of porous carbon composite membrane would become a nanostructure matrix extensively applied in the field of oxygen reduction sensors
2. Experimental 2.1. ChemicalsFilter paper was bought from Hangzhou Xinhua paper (Hangzhou,China). Nafion (5.0 wt%) were purchased from Sigma-Aldrich. Iron nitrate and methanol were bought from Beijing Chemical Reagent Company (Beijing,China). All the chemicals were analytical grade,and used as received. All aqueous solutions were prepared with ultrapure water from a Water Purifier System (Sichuan Water Purifier Co.,Ltd.,China).
2.2. ApparatusElectrochemical measurements were performed on a CHI 660 Electrochemical Analyzer (Co.,CHI,USA) with a conventional three-electrode system comprised of platinum wire as auxiliary electrode,an Ag/AgCl (saturated KCl) electrode as the reference electrode and glassy carbon electrode (GCE,diameter 3 μm) as working electrode. Rotating ring-disk electrode (RRDE) techniques were employed on a Model RRDE-3A Apparatus (ALS,Japan) with a CHI840B Electrochemical Workstation. Scanning electron microscopy (SEM) images with an accelerating voltage of 20 kV were obtained on an XL 30 ESEM FEG SEM (Philips,Netherlands). Transmission electron microscopy (TEM) measurements were made on a HITACHI H-8100 EM with an accelerating voltage of 100 kV. X-ray photoelectron spectroscopy (XPS) analysis was obtained on an ESCALABMKII X-ray photoelectron spectrometer (VG Scientific,UK).
2.3. Preparation of porous carbon composite membrane and electrodeThe commercial filter paper was stabilized at 240 ℃ for 2 h in a muffle furnace at a heating rate of 2 ℃/min. Then the material was impregnated with a known concentration of Fe(NO3)3,and dried at room temperature. Finally,the catalyst was prepared by temperature-programming in a tube furnace under N2 at a heating rate of 2 ℃/min,The final product was based on the pyrolysis temperature (900 ℃) and concentration of Fe(NO3)3 (1.0%),respectively [11]. Before modification,the GCE was polished carefully using 0.3 μm alumina slurries,followed by sonication successively in acetone,ethanol and ultrapure water,and then dried at room temperature. For a typical procedure,4.0 mg of the PCCM sample were dissolved in a mixture (4 ml) of water,isopropyl alcohol,and Nafion (5.0 wt%) at a ratio of 20:1:0.75 (v/v/v) under sonication. For the electrochemical measurements,a certain amount of the PCCM suspension was coated onto the pretreated GCE surface,and then the PCCM/GCE was dried under an infrared lamp before use.
2.4. Electrochemical measurementsThe cyclic voltammetric and amperometric experiments were performed in a thermostated electrochemical cell at 25 ℃. Amperometric experiments were carried out in 0.1 mol/L KOH solution with stirring by applying a potential step of -0.29 V. When the current reached a steady-state value,substrate was added at one sample per 80 s. Different volumes of an O2-saturated solution were spiked into N2-saturated 0.1 mol/L KOH for the detection of dissolved oxygen. In the RRDE experiments,linear sweeping voltammograms (LSVs) were obtained by performing a negative-direction sweep of potential from 0.2 V at a rate of 5 mV/s, with the ring potential set at 0.2 V in 0.10 mol/L KOH. Before beginning experiments,all the modified electrodes and the Pt ring electrode were activated by potential cycling in 0.10 mol/L KOH from 0.2 V to -0.8 V at a scan rate of 50 mV/s for 30 cycles.
3. Results and discussion 3.1. Physical and chemical characterization of porous carbon composite membrane electrodeThe transmission electron microscopy (TEM) results (Fig. 1A) of these materials are consistent with the SEM images. The transmission electron microscopy (TEM) of the porous carbon composite membrane exhibited a relative homogeneous morphology with uniformly dispersed nanoparticles near,or,in the pores, and the diameters of the particles ranging from 30 nm to 100 nm. Because the oxidative gases,such as NO2,O2,and NO,will be released when Fe(NO3)3 melts at high temperatures [27],there are some pores which appeared on the surface of the PCCM. The highresolution TEM images shown in Fig. 2 are taken from the PCCM. The distances of 0.21 nm and 0.376 nm are corresponding to (2 1 1) and (0 1 1) crystal planes of Fe3C phase,respectively,and the Fe3C nanoparticles are surrounded by the carbon shells [5, 28].

|
Download:
|
| Fig. 1. TEM image of (A) PCCM and (B) SEM image of PCCM. | |

|
Download:
|
| Fig. 2. High resolution TEM images of the PCCM. The distances of 0.21 nm (A) and 0.376 nm (B) correspond to (2 1 1) and (0 1 1) crystal planes of Fe3C phase, respectively. | |
The XPS analysis can provide additional information about the oxygen-containing surface groups. The XPS analysis indicates the PCCM is mainly composed of C (284.6 eV),O (532.5 eV),and Fe (711.5 eV),confirming that Fe has been successfully doped into the carbon composite (Fig. 3A). In detail,the spectra of Fe 2p (Fig. 3B) of the PCCM reveal the distribution of Fe 2p into two species: Fe 2p3/2 and Fe 2p1/2 observed at 710.5 eV and 723.9 eV,respectively,from which we can deduce that iron atoms in the catalyst are either in the metallic,oxidation,or carbidic state [28]. Recent studies have verified that the encased Fe3C activates the surrounding graphitic layers,making the outer surface of the carbon layer active toward the ORR [29]. Therefore,the as-prepared PCCM with evenly dispersed Fe3C nanoparticles should find application in the ORR.
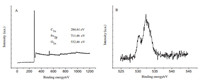
|
Download:
|
| Fig. 3. (A) XPS survey for the resultant PCCM and (B) high-resolution Fe2p spectra. | |
In the potential window from +0.2 V to -0.8 V,after bubbling the detection solution thoroughly with high purity nitrogen for 5 min,the PCCM electrode began to reduce dissolved oxygen at about -0.15 V in 0.10 mol/L KOH. The cyclic voltammograms showed an obvious reduction peak centered at about -0.29 V (Fig. 4),while at the bare GCE a negative shift of the smaller reductive peak was observed in the scan range. Compared to other modified electrodes,the PCCM modified glassy carbon electrode displays superior ORR activity which has more positive reduction peak potential,onset potential,and higher reduction current density in O2-saturated 0.10 mol/L KOH.
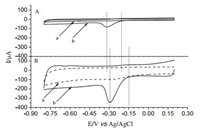
|
Download:
|
| Fig. 4. Cyclic voltammograms of Bare GCE (A) and PCCM/GCE (B) in N2-saturated 0.1 mol/L KOH (a) and O2-dissolved 0.1 mol/L KOH (b) scan rate is 50 mV/s (vs Ag/AgCl). | |
The kinetics of ORR of the PCCM/GCE was further studied by linear sweeping voltammograms at 1600 rpm using rotating ring disk electrode (RRDE). Notably,the number of transferred electrons (n) is calculated from the ring and disk currents,and is observed to be 3.82-3.97 over the entire investigated potential range (Fig. 5A). As shown in Fig. 5B,the H2O2 yield of the PCCM/ GCE is below 17.3% over the potential range of -0.2 to -0.65 V in O2-saturated 0.10 mol/L KOH based on the assumption that oxygen reacts in a parallel mechanism [30]. All of these conclusions indicate that the fabricated PCCM/GCE possesses superior electrocatalytic ORR activity and a four-electron transfer process is predominant.

|
Download:
|
| Fig. 5. (A) RRDE voltammograms of PCCM/GCE in O2-saturated 0.10 mol/L KOH at a scan rate of 5 mV/s, a rotation rate of 1600 rpm. Inset: electron transfer number (n) and (B) H2O2 yield of PCCM/GCE. | |
The analytical performance of the sensor was verified by amperometry. In these measurements,an initial study was performed in order to determine the best potential to be applied to the electrode. The applied potential was chosen based on measurements of the catalytic current using the optimized conditions and the highest current verified at an applied potential of -0.29 V versus Ag/AgCl. The amperometric trace recorded at -0.29 V of the PCCM electrode displayed in Fig. 6 during the spiking of different volumes of O2-saturated solution into N2- saturated KOH solution,illustrates that the modified electrode responds very rapidly to these changes in the O2 concentration, producing steady-state signals within 10 s. The response displayed a linear range from 2 μmol/L to 110 μmol/L with a correlation coefficient of 0.999. The limit of detection is 1.4 μmol/L at the signal to noise ratio of 3. The high sensitivity is attributed to the excellent catalytic activity of uniformly dispersed Fe3C nanoparticles of the membrane. Repeated use of the electrodes did not affect the long-term stability. The coefficients of variation of the current signals for ten repeated injections of two and 20 μmol/L of O2 were 2.6 and 3.0%,respectively. The modified electrode showed acceptable preparation reproducibility with a relative standard deviation of 3.0% estimated from the slopes of the calibration plots on eight freshly prepared PCCM/GC electrodes. When the modified electrode was stored at room temperature no significant change in the response was observed after more than 2 months.

|
Download:
|
| Fig. 6. Typical steady-state current response of the PCCM/GCE to successive additions of different volumes of O2-saturated solution into N2-saturated 0.1 mol/L KOH. Applied potential, -0.29 V. Inset: calibration curve of the electrocatalytic current on the concentration of oxygen. | |
The effects of substances that might interfere with the response of the PCCM electrode were studied (Table 1). The inhibition current obtained for each interfering substance present at a concentration of 110 μmol/L was compared with the current obtained in the presence of 110 μmol/L O2,and this ratio was used as a criterion for the selectivity of the sensor. As can be verified,the observed influences are all less than 3% on the dissolved oxygen response. These results reveal that the fabricated sensor can tolerate high concentrations of interfering species and,therefore, can be claimed as selective in the presence of the commonly encountered foreign species.
| Table 1 Inhibition of the interferences to the PCCM/GC electrode. |
We have demonstrated a simple method for the fabrication of an oxygen reduction sensor based on a novel PCCM/GC electrode from renewable,low-cost filter paper. The PCCM electrode shows excellent electrocatalytic activity toward the electroreduction of dissolved oxygen and can thus be used for highly sensitive detection of dissolved oxygen. The results indicated that the porous structure and the Fe-based nanoparticles greatly improved the electrocatalytic ORR activity of the PCCM electrode. Therefore, the successful fabrication of PCCM/GCE is more amenable to largescale production due to the cheap carbon precursor and the simple, two-step synthetic process for the oxygen reduction sensor.
AcknowledgmentsWe acknowledge financial supports from the National Natural Science Foundation of China (No. 21273097),the project from the State Key Laboratory of Electroanalytical Chemistry (No. 2013) and the Science Foundation of Jilin Province (No. 20130204003GX).
| [1] | R.Martínez-Máñez,J.Soto,J.Lizondo-Sabater,et al.,New potentiomentric dissolved oxygen sensors in thick film technology,Sens.Actuators B:Chem.101(2004)295-301. |
| [2] | S.Shanmugam,T.Osaka,Efficient electrocatalytic oxygen reduction over metal free-nitrogen doped carbon nanocapsules,Chem.Commun.47(2011)4463-4465. |
| [3] | A.Morozan,P.Jégou,S.Campidelli,S.Palacin,B.Jousselme,Relationship between polypyrrole morphology and electrochemical activity towards oxygen reduction reaction,Chem.Commun.48(2012)4627-4629. |
| [4] | Y.Hu,J.O.Jensen,W.Zhang,et al.,Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts,Angew.Chem.Int.Ed.53(2014)3675-3679. |
| [5] | Y.F.Zhang,X.J.Bo,C.Luhana,et al.,Facile synthesis of a Cu-based MOF confined in macroporous carbon hybrid material with enhanced electrocatalytic ability,Chem.Commun.49(2013)6885-6887. |
| [6] | Y.M.Tan,C.F.Xu,G.X.Chen,et al.,Facile synthesis of manganese-oxide-containing mesoporous nitrogen-doped carbon for efficient oxygen reduction,Adv.Funct.Mater.22(2012)4584-4591. |
| [7] | Z.Y.Zhang,G.M.Veith,G.M.Brown,et al.,Ionic liquid derived carbons as highly efficient oxygen reduction catalysts:first elucidation of pore size distribution dependent kinetics,Chem.Commun.50(2014)1469-1471. |
| [8] | J.T.Jin,F.P.Pan,L.H.Jiang,et al.,Catalyst-free synthesis of crumpled boron and nitrogen Co-doped graphite layers with tunable bond structure for oxygen reduction reaction,ACS Nano 8(2014)3313-3321. |
| [9] | S.K.Ramasahayam,U.B.Nasini,V.Bairi,A.U.Shaikh,T.Viswanathan,Microwave assisted synthesis and characterization of silicon and phosphorous Co-doped carbon as an electrocatalyst for oxygen reduction reaction,RSC Adv.4(2014)6306-6313. |
| [10] | L.T.Qu,Y.Liu,J.-B.Baek,L.M.Dai,Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells,ACS Nano 4(2010)1321-1326. |
| [11] | S.M.Zhang,H.Y.Zhang,Q.Liu,S.L.Chen,Fe-N doped carbon nanotube/graphene composite:facile synthesis and superior electrocatalytic activity,J.Mater.Chem.A 1(2013)3302-3308. |
| [12] | K.I.Ozoemena,S.A.Mamuru,T.Fukuda,N.Kobayashi,T.Nyokong,Metal (Co,Fe) tribenzotetraazachlorin-fullerene conjugates:impact of direct π-bonding on the redox behaviour and oxygen reduction reaction,Electrochem.Commun.11(2009)1221-1225. |
| [13] | Y.Wang,Y.Y.Shao,D.W.Matson,J.H.Li,Y.H.Lin,Nitrogen-doped graphene and its application in electrochemical biosensing,ACS Nano 4(2010)1790-1798. |
| [14] | W.Ding,Z.D.Wei,S.G.Chen,et al.,Space-confinement-induced synthesis of pyridinic-and pyrrolic-nitrogen-doped graphene for the catalysis of oxygen reduction,Angew.Chem.Int.Ed.52(2013)11755-11759. |
| [15] | X.J.Bo,L.P.Guo,Ordered mesoporous boron-doped carbons as metal-free electrocatalysts for the oxygen reduction reaction in alkaline solution,Phys.Chem.Chem.Phys.15(2013)2459-2465. |
| [16] | J.J.Duan,Y.Zheng,S.Chen,et al.,Mesoporous hybrid material composed of Mn3O4 nanoparticles on nitrogen-doped graphene for highly efficient oxygen reduction reaction,Chem.Commun.49(2013)7705-7707. |
| [17] | G.Wu,K.L.More,C.M.Johnston,P.Zelenay,High-performance electrocatalysts for oxygen reduction derived from polyaniline,iron,and cobalt,Science 332(2011)443-447. |
| [18] | H.Zhu,J.Yin,X.L.Wang,H.Y.Wang,X.R.Yang,Microorganism-derived heteroatom-doped carbon materials for oxygen reduction and supercapacitors,Adv.Funct.Mater.23(2013)1305-1312. |
| [19] | R.J.White,V.Budarin,R.Luque,J.H.Clark,D.J.Macquarrie,Tuneable porous carbonaceous materials from renewable resources,Chem.Soc.Rev.38(2009)3401-3418. |
| [20] | L.Wang,Q.Y.Zhang,S.L.Chen,et al.,Electrochemical sensing and biosensing platform based on biomass-derived macroporous carbon materials,Anal.Chem.86(2014)1414-1421. |
| [21] | Y.L.Zhai,C.Z.Zhu,E.K.Wang,S.J.Dong,Energetic carbon-based hybrids:green and facile synthesis from soy milk and extraordinary electrocatalytic activity towards ORR,Nanoscale 6(2014)2964-2970. |
| [22] | C.Z.Zhu,J.F.Zhai,S.J.Dong,Bifunctional fluorescent carbon nanodots:green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction,Chem.Commun.48(2012)9367-9369. |
| [23] | W.X.Yang,Y.L.Zhai,X.Y.Yue,Y.Z.Wang,J.B.Jia,From filter paper to porous carbon composite membrane oxygen reduction catalyst,Chem.Commun.50(2014)11151-11153. |
| [24] | H.S.Zhai,L.Cao,X.H.Xia,Synthesis of graphitic carbon nitride through pyrolysis of melamine and its electrocatalysis for oxygen reduction reaction,Chin.Chem.Lett.24(2013)103-106. |
| [25] | W.X.Yang,X.J.Liu,X.Y.Yue,J.B.Jia,S.J.Guo,Bamboo-like carbon nanotube/Fe3C nanoparticle hybrids and their highly efficient catalysis for oxygen reduction,J.Am.Chem.Soc.137(2015)1436-1439. |
| [26] | M.Sobiesiak,Nanoporous carbons obtained by carbonization of copolymers impregnated by salts,Adsorption 19(2013)349-356. |
| [27] | J.Su,Y.H.Gao,R.C.Che,Synthesis and microstructure of Fe3C encapsulated inside chain-like carbon nanocapsules,Mater.Lett.64(2010)680-683. |
| [28] | J.Fournier,G.Lalande,R.Coté,D.Guay,J.P.Dodelet,Activation of various Febased precursors on carbon black and graphite supports to obtain catalysts for the reduction of oxygen in fuel cells,J.Electrochem.Soc.144(1997)218-226. |
| [29] | M.Bron,P.Bogdanoff,S.Fiechter,et al.,Influence of selenium on the catalytic properties of ruthenium-based cluster catalysts for oxygen reduction,J.Electroanal.Chem.500(2001)510-517. |
 2015, Vol.26
2015, Vol.26 



