b The Ural Federal University Named after the First President of Russia B. N. Yeltsin, Ekaterinburg 620002, Russia
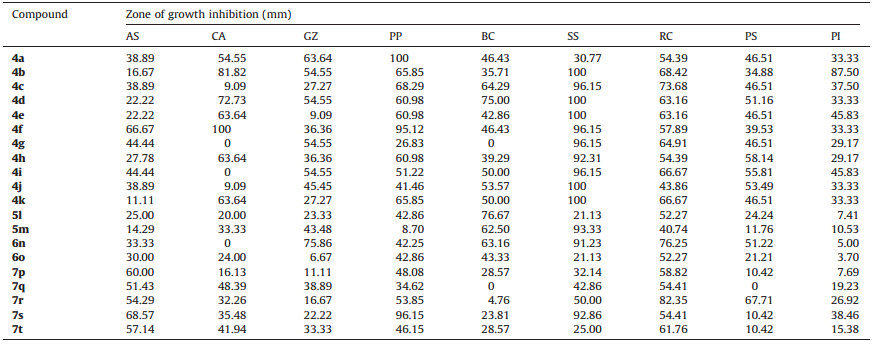
The application of elicitors is a novel,modern measure for pesticide development and environmental protection because elicitors can induce the immunological system of the plant to provide a broad spectrum of systemic acquired resistance at the physical and physiological level of the host plants[1].Compound A (Fig.1) had good systemic acquired resistance activity,which presented a better antimicrobial biology than metsulfovax (Fig.1) [2].Both of them belong to the derivatives of 1,3-thiazole.1,3- Thiazoles are important heterocyclic compounds with low toxicity to mammals and a broad-spectrum of biological activities including insecticidal[3],antifungal[4, 5],herbicidal[6, 7],regulating plant growth[8, 9],and antiviral activities[10].Many thiazole derivatives such as thiamethoxam[11],imidaclothiz,thiabendazole[12],and benthiavalicarbisopropyl[13]had been commercialized as agrochemicals.In addition,the compounds with an amide or ester group were a versatile class of agrochemicals with wide range of biological activities[14]and have been used as insecticidal[15, 16],fungicidal[17, 18],herbicidal[19],and antiviral agents[2].To find novel pesticide candidates with a wide spectrum of biological activities,especially systemic acquired resistance,a series including 20 novel 2-amino- 1,3-thiazole-4-carboxylic acid derivatives were designed and synthesized for biological screening according to bioactive substructure coordination strategy.
|
Download:
|
| Fig. 1. The structure of compound A and Metsufovax. | |
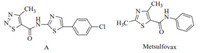
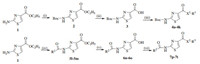
Reagents were all analytically or chemically pure.All the solvents and liquid reagents were dried by standard methods in advance and distilled before use.Synthesis of the title compounds was conducted as shown in Scheme 1 and the structure of the title compounds was shown in Fig.2.The starting material,ethyl 2- aminothiazole-4-carboxylate (compound 1) was prepared according to the literature[20, 21].Intermediate 2 was obtained through protection of amine group with di-tert-butyl dicarbonate (Boc2O); the hydrolysis of intermediate 2 with NaOH gave acid 3;the title compounds 4a-4k were obtained by condensation reaction using Et3N as the base and N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDCI)/1-hydroxybenzotriazole (HOBT) as condensation agents;compounds 5l-5m were obtained through the same manner of compounds 4a-4k;compounds 6n-6o were synthesized by hydrolysis of ester 5l-5m with NaOH;compounds 7p-7t were obtained by condensation reaction using Et3N as the base and EDCI/HOBT as condensation agents.
|
Download:
|
| Scheme 1. Reagents and conditions:(i) Boc2O (1.0 equiv.),DMAP (0.018 equiv.),CH2Cl2,room temperature (r.t.) for 4 h,yield 93.5%;(ii) NaOH (1 equiv.),H2O,CH3OH/ THF=1:1(v/v),r.t.for 3 h,yield 80.5%;(iii) R1NH2 or R1OH (0.95 equiv.),EDCI (1.14 equiv.),HOBT (1.0 equiv.),Et3N (1.14 equiv.),CH2Cl2,r.t.for overnight,yield 50.1%-86.4%; (iv)2-(5-methyl-3-(trifluoromethyl)-1 H-pyrazol-1-yl) acetic acid or 3-bromo-1-(3-chloropyridin-2-yl)-1 H-pyrazole-5-carboxylic acid (1 equiv.),EDCI (1.14 equiv.),HOBT (1.0 equiv.),Et3N (1.14 equiv.),CH2Cl2,r.t.for overnight,yield 41.0%-45.2%;(v) NaOH (1 equiv.),H2O,CH3OH/THF=1:1(v/v),r.t.for 3 h,yield 60.0%-89.0%;(vi) R3NH2 or R3OH (0.95 equiv.),EDCI (1.14 equiv.),HOBT (1.0 equiv.),Et3N (1.14 equiv.),CH2Cl2,r.t.for overnight,70.1%-89.0%. | |

|
Download:
|
| Fig. 2. The structure of the title compounds. | |
The structures of all newly synthesized compounds were characterized by melting points,IR,1H NMR,13C NMR,and HRMS or elemental analysis. The fungicidal activity determination was conducted by fungi growth inhibition method according to the reference using potato dextrose agar (PDA) as the cultivation medium[22].Fungi used in this study included Alternaria solani (AS),Botrytis cinerea (BC),Cercospora arachidicola (CA),Gibberella zeae (GZ),Phytophthora infestans (Mont) de Bary (PI),Physalospora piricola (PP),Pellicularia sasakii (PS),Sclerotinia sclerotiorum (SS),and Rhizoctonia cerealis (RC).The antivirus activity against TMV for curative,inactive,inductive,and protective models was also tested according to the literature[23],Tiadinil,Ningnanmycin,and Virazol were used as positive controls.Supplementary materials,including experimental procedures and the physicochemical data of the title compounds,associated with this article can be found in the Supporting Information.
3.Results and discussionAll IR data showed strong carbonyl group absorptions at 1750- 1550 cm-1,and absorptions at about 3390-2850 cm-1 were observed from the NH group.In the 1H NMR spectra,the chemical shift of a proton in the thiazole ring of all target compounds was observed at δ 7.49-8.05,nine protons of tert-butyl group were observed at δ 1.56-1.44 as a single peak.In the 13C NMR spectra,the C55O peaks were observed at δ 170-150,the thiazole-C were at δ 165-110,the C of CHF2 was a triple peak at δ 110-116 and the CH2 of CH2CHF2 also was a triple peak at δ 40-42.The elemental analysis data or HRMS spectral data of all compounds were in good agreement with the calculated value.
The fungicidal activity data in Table 1 indicated that the title compounds had a broad spectrum of fungicidal activity.The majority of them had good fungicidal activity against PP,SS,and RC with growth inhibition over 50%.All compounds except 4j and 5m had good fungicidal activity against RC with growth inhibition over 50%.Compounds 4b and 4i exhibited over 50% activity against six fungi tested and compound 4d exhibited over 50% activity against seven fungi tested.Compounds 4a,4b,4d,4e,4f,4j,and 4k stood out with growth inhibition at 100% against PP,SS,SS,SS,CA,SS,and SS,respectively.
| Table 1 Fungicidal activity of test compounds (%, 50 μg/mL). |
Antiviral activities of all title compounds against TMV in vivo were evaluated,and the results were shown in Table 2.As can be seen from these data,compounds 4e,4g,4h,4k,and 7p had good curative activity against TMV at 100 μg/mL with inhibitions over 50%,which were higher than that of the positive control Ningnanmycin.Compounds 4a,4c,4e,4f,4g,4h,4j,5l,6n,6o,7r,7s,and 7t had good inactivation activity against TMV with inhibition rates over 50%,which were higher than that of the positive control,Ningnanmycin.Compounds 4c,4d,4e,4k,6o,and 7p had good induction activities of tobacco against TMV over 80%,which were higher than that of the positive control Tiadinil. Compounds 4b,4c,4d,4e,4h,4i,4k,6n,6o,7q,7r,and 7s exhibited excellent protection activity with inhibitions of 60.95%,89.52%,68.57%,87.62%,74.29%,62.86%,82.86%,84.29%,67.14%,90.48%,77.14%,and 77.14%,respectively,which were higher than the positive control Virazol.Compounds 4c and 4e stood out showing high effects against TMV in vivo in all four models.These studies presented an important basis for development of a novel pesticide with both fungicidal and antivirus activity.
| Table 2 Antiviral activity of the test compounds against TMV (%, 100 μg/mL). |
In summary,a series of novel 2-amino-1,3-thiazole-4-carboxylic acid derivatives were designed and synthesized with starting material ethyl 2-aminothiazole-4-carboxylate.The bioassay indicated that most of the title compounds had good fungicidal activity at 50 μg/mL and anti-TMV activity in vivo at 100 μg/mL.The results suggested that the novel 2-amino-1,3-thiazole-4-carboxylic acid was an important substructure for novel pesticide development.
AcknowledgmentsThis work was partially supported by The International Science &Technology Cooperation Program of China (No.2014dFR41030). Nataliya P.Belskaya thanks Russian State Task of Ministry Education and Science (No.4.560.2014/K).Kalinina Tatiana thanks RFBF (No.13-03-00137).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.05. 040.
| [1] | Z.J.Fan,X.F.Liu,F.L.Liu,L.L.Bao,Y.G.Zhang,Progress of researches on induced resistance of plant activator,Acta Phytophy.Sin.32(2005)87-92. |
| [2] | Z.J.Fan,Z.G.Shi,H.K.Zhang,et al.,Synthesis and biological activity evaluation of 1,2,3-thiadiazole derivatives as potential elicitors with highly systemic acquired resistance,J.Agric.Food Chem.57(2009)4279-4286. |
| [3] | C.L.Liu,L.Li,Z.M.Li,Design,synthesis,and biological activity of novel 4-(3,4-dimethoxyphenyl)-2-methylthiazole-5-carboxylic acid derivatives,Bioorg.Med.Chem.12(2004)2825-2830. |
| [4] | A.R.Jalilian,S.Sattari,M.Bineshmarvasti,A.Shafiee,M.Daneshtalab,Synthesis and in vitro antifungal and cytotoxicity evaluation of thiazolo-4H-1,2,4-triazoles and 1,2,3-thiadiazolo-4H-1,2,4-triazoles,Arch.Pharm.Med.Chem.333(2000)347-354. |
| [5] | S.Vengurlekar,S.Prachand,S.Jain,R.Gupta,Synthesis and evaluation of some thiazole derivatives as an antifungal agent,Int.J.Pharm.Life Sci.5(2014)3526-3530. |
| [6] | T.T.Wang,G.F.Bing,X.Zhang,et al.,Synthesis and herbicidal activities of 2-cyano-3-benzylaminoacrylates containing thiazole moiety,Bioorg.Med.Chem.Lett.20(2010)3348-3351. |
| [7] | H.Dai,Y.S.Xiao,Z.Li,X.Y.Xu,X.H.Qian,The thiazoylmethoxy modification on pyrazole oximes:synthesis and insecticidal biological evaluation beyond acaricidal activity,Chin.Chem.Lett.25(2014)1014-1016. |
| [8] | X.Qin,H.B.Yu,H.Dai,et al.,Synthesis and plant-growth regulatory activities of novel imine derivatives containing 1H-1,2,4-triazole and thiazole rings,Chin.Chem.Lett.21(2010)283-286. |
| [9] | H.B.Yu,L.Shao,J.X.Fang,Synthesis and biological activity research of novel ferrocenyl-containing thiazole imine derivatives,J.Organomet.Chem.692(2007)991-996. |
| [10] | A.S.Mayhoub,M.Khaliq,C.Botting,et al.,An investigation of phenylthiazole antiflaviviral agents,Bioorg.Med.Chem.19(2011)3845-3854. |
| [11] | P.Maienfisch,M.Angst,F.Brandl,et al.,Chemistry and biology of thiamethoxam:a second generation neonicotinoid,Pest Manag.Sci.57(2001)906-913. |
| [12] | M.H.Lee,S.M.Pan,T.W.Ng,et al.,Mutations of β-tubulin codon 198 or 200 indicate thiabendazole resistance among isolates of Penicillium digitatum collected from citrus in Taiwan,Int.J.Food Microbiol.150(2011)157-163. |
| [13] | H.C.Feng,Novel fungicide benthiavalicarbisopropyl,World Pestic.30(2008)51. |
| [14] | J.Huang,D.Wu,H.J.Ge,S.H.Liu,J.Yin,Fluorinated 1,8-naphthalimides:synthesis,solid structure and properties,Chin.Chem.Lett.25(2014)1399-1402. |
| [15] | C.L.Liu,Z.M.Li,B.Zhang,synthesis and biological activity of novel 2-methyl-4-trifluoromethyl-thiazole-5-carboxamide derivatives,J.Fluorine Chem.125(2004)1287-1290. |
| [16] | Y.B.Chen,J.L.Li,X.S.Shao,X.Y.Xu,Z.Li,Design,synthesis and insecticidal activity of novel anthranilic diamides with benzyl sulfide scaffold,Chin.Chem.Lett.24(2013)673-676. |
| [17] | Z.Y.Yu,G.Y.Shi,Q.Sun,et al.,Design,synthesis and in vitro antibacterial/antifungal evaluation of novel 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7(1-piperazinyl) quinoline-3-carboxylic acid derivatives,Eur.J.Med.Chem.44(2009)4726-4733. |
| [18] | C.B.Vicentini,C.Romagnoli,E.Andreotti,D.Mares,Synthetic pyrazole derivatives as growth inhibitors of some phytopathogenic fungi,J.Agric.Food Chem.55(2007)10331-10338. |
| [19] | H.Cerecetto,E.Dias,R.D.Maio,et al.,Synthesis and herbicidal activity of N-oxide derivatives,J.Agric.Food Chem.48(2000)2995-3002. |
| [20] | V.Ehmke,J.E.Q.Quinsaat,P.Rivera-Fuentes,et al.,Tuning and predicting biological affinity:aryl nitriles as cysteine protease inhibitors,Org.Biomol.Chem.10(2012)5764-5768. |
| [21] | N.P.Belskaya,K.I.Lugovik,A.D.Ivina,V.A.Bakulev,Z.J.Fan,Reaction of enamines and azaenamines containing a thioamide group with dimethyl acetylenedicarboxylate,Chem.Heterocycl.Compd.50(2014)888-900. |
| [22] | Z.J.Fan,Z.K.Yang,H.K.Zhang,et al.,Synthesis,crystal structure,and biological activity of 4-methyl-1,2,3-thiadiazole-containing 1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles,J.Agric.Food.Chem.58(2010)2630-2636. |
| [23] | Y.D.Li,W.T.Mao,Z.J.Fan,et al.,Synthesis and biological evaluation of novel 1,2,4-triazole containing 1,2,3-thiadiazole derivatives,Chin.Chem.Lett.24(2013)1134-1136. |
 2015, Vol.26
2015, Vol.26