Ionic liquids (ILs) are favourable in various chemical applications due to their unique physiochemical properties. ILs are generally known to possess undetectable vapour pressure,wide liquid temperature range,high solubility for many organic and inorganic compounds,and low toxicity [1, 2, 3]. Hence,ILs are applied in many chemical and industrial processes such as CO2 capture [4, 5, 6],battery development [7, 8],electrochemical applications [9] and biocatalysts [10, 11],organic synthesis [12],material synthesis [13],extraction [5],and biomedical applications [5].
Deep eutectic solvents (DESs) are an alternative class of ILs resembling room temperature ionic liquids (RTILs),they are distinguished by the fact that they contain an organic molecular component that play a role as the predominant constituent [14]. DESs are of great interest as an alternative to RTILs owing to their remarkable qualities given as tolerance to humidity,negligible vapour pressure thus non-flammable,high thermostability,less expensive,non-toxic,reusable and biodegradable as compared with their RTIL derivatives [1, 2, 15, 16, 17]. However,the classification of biodegradable and environmentally of DESs depends greatly on the selected constituents [18].
Research done on DESs are mainly limited to the most common choline based DESs. They are rather well studied,vastly synthesized and are widely employed in various industrial uses such as sugar industries [10],electropolishing,electroplating,metal oxide processing [9, 19, 20, 21] and fabrication of nanomaterial [22, 23]. Study on type IV DESs (metal-based) is scarcely explored. A wide range of DESs should be synthesized,developed and be applied in order to fulfil the needs of a diverse field in solvent chemistry. The main interest in this study is to attempt manganese (II)-based DESs synthesized in combination with different HBDs. Following that,characterization of physical properties on DESs obtained such as investigations on intermolecular interaction via FTIR,viscosity,conductivity and thermal stability analyses were further implemented. The findings obtained would reveal the characteristics,nature or features of these DESs as potential industrial solvents.
2. Experimental 2.1. Direct heating and evaporating methodsDirect heating method was carried out as according to method extracted from Dai et al. [24]. Procedures were executed in two parts,one with fixed amount (2.0 g) of MnCl2-4H2O and varying amount (0.4,0.8,1.2,1.6 and 2.0 g) of hydrogen bond donors (HBDs) and vice versa. A graph of freezing points against calculated mole ratios of MnCl2-4H2O/HBD in each composition was plotted (Fig. S1 and Table S1 in Supporting information). The mole ratio of the potential DESs was marked from the intersections from the graph.
Evaporating method was applied for the liquid mixture with thermally sensitive HBDs such as D-glucose and D-fructose as method published by Dai et al.,except that water was replaced by methanol [24]. The amount of both MnCl2-4H2O and HBD was calculated and weighed based on the predicted moleratio (2:1,1:1,and 1:2). Methanol was evaporated off from the homogenous liquid by gentle heating and the mixture was weighed from time to time until a constant weight was obtained.
2.2. Synthesis of DESsPotential DESs were prepared according to the predetermined mole ratios obtained either by using the graph of freezing point against calculated mole ratios or the predicted possible mole ratios from direct heating and evaporating methods. These eutectic mixtures were kept for observation across a period of 14 days at room temperature in a desiccator as their sensitivity to moisture by nature might affect their physiochemical properties [25]. If precipitation or turbidity is not observed in these eutectic mixtures,DES is successfully obtained. The synthesized DESs were used without any further purification.
2.3. Characterizations of DESsFourier Transform Infrared Spectroscopy (FT-IR) analysis of DESs was carried out by using 100 SERIES Perkin-Elmer (USA) with Universal Attenuated Total Reflectance (UATR) technique. The absorption bands were measured in the range of 4000-280 cm-1. To check the thermal stability,thermogravimetric analysis (TGA) of all DESs obtained were investigated by using Perkin Elmer Simultaneous Thermal Analyser (STA 6000) (USA) under nitrogen environment. Temperature of instrument was set ranging from room temperature to 1000 ℃ under nitrogen atmosphere for 10 ℃/min. The viscosity of all DESs was measured by using Brookfield LV DV-II+ Pro Viscometer (USA). The viscometer was set up and calibrated for every usage for different samples. Conductivity analysis was performed by using a conductivity meter S30 (SevenEasy,USA). Conductivity ranging from 0.01 μS/cm to 500 μS/cm was subjected for auto scaling. The meter was calibrated with conductivity standard of 1413 μS/cm. The conductivity accuracy is ±0.5% of the value shown.
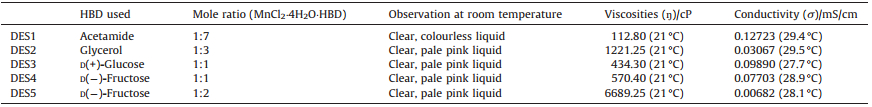
3. Results and discussionFive manganese (II)-based DESs were successfully synthesized in this study. The successfully synthesized DESs with their eutectic compositions are listed in Table 1. In direct heating approach,only DES1 andDES2remainedas clearliquidformformorethan 14days at room temperature. Unsuccessful combinations appear as white semisolid precipitation upon cooling down to room temperature. On the other hand,evaporating method successfully synthesized DES3,DES4 and DES5. It was reported that compounds that can form best ILs are those possessing lower freezing points [26]. In this study,freezing points of individual DES were found as refer to the mole ratio of each potent DES obtained from direct heating method. DES1 was found to exhibit a depression of freezing point at 27.5 ℃,which is noticeably lower than both original components - MnCl2-4H2O (81 ℃) and acetamide (58 ℃).
| Table 1 Composition, abbreviations and observations of the studied DESs with different HBDs. |
DES2 possesses a depression of freezing point at 34.5 ℃; the depression is clearly not as much as of DES1,possibly owing to the formation of covalent bonds. As the covalent bonds were formed,the crystal structure of MnCl2-4H2O with acetamide or glycerol collapsed and this could leads towards the lower lattice energies in the DESs [27]. Where in such case,lesser energy is required to break the bonds,resulting in a depression of freezing point when the ion size is larger with smaller ion charges [28]. Thus,such DES with low lattice energy is the reason for its liquid phase existence at room temperature [27]. These findings are especially interesting as generally,DESs with a freezing point lower than 50 ℃ are of special demand since they could be at lower cost of production and usage,also as safer solvents in many industries [29]. Hence,the clear liquid yields of DES1,DES2,DES3,DES4,and DES5 were selected for further investigations.
3.1. FTIR spectrums analysisFourier Transform Infrared Spectroscopy (FTIR) is employed to analyze the structure of different chemical groups and their interactions. Interpretation of DES structures can be done by doing the analysis of the frequency shifts,bandwidths and absorbance values of the same bond in different mixture systems [30]. The synthesized DESs with relevant IR absorption bands are illustrated in Fig. S1.
The broad peak between 3500 and 3000 cm-1 obtained shows the hydrogen bond forming in all five synthesized DESs. The hydrogen bonds formed in the system of DES1 are N-H···OH between amine group of acetamide and hydrated manganese salt,while the hydrogen bonds formed both in the DES2,DES3,DES4 and DES5 are the H-O···H. It is detected as broader bands in DES4 and DES5 at 3292 cm-1 and 3275 cm-1 respectively,which is found at lower wavenumber. The differences for these to occur mainly due to the participation of sugar OH groups in metal- ligand bonding interaction,which most probably with the coordinated OH2 from MnCl2-4H2O [31, 32]. The broader peak of OH stretching band and the disappearance of NH2 wags of acetamide (υ = 674 cm-1) in spectrum of DES1 provides the evidence of the formation of hydrogen bonding. The minor shifting frequency of υC=O in DES1 indicates that the C=O···H-O is formed. The weak peak of alcohol C-O stretching between 1200 and 750 cm-1 in DES2,is shifted to 1032 cm-1 also shows the hydrogenbonds H-O···Hexists.For DES2,DES3,DES4andDES5,a broad band of OH wags in alcohol and water was obviously found at 900-500 cm-1 and it also implies the existence of hydrogen bonds. Theoretically,there are possibilities that chlorine atom (Mn-Cl) could also act as hydrogen-bond acceptors [33]. However,IR spectrums obtained showed no noticeable signal for chlorine interactions with any other atoms. Nonetheless,the next step would be the application of technique such as X-ray crystallography to provide further evidence supporting all the above speculations.
3.2. Thermal behaviour of synthesized DESsTGA is used to determine selected characteristics of DESs that underwent either mass loss or gain owing to decomposition,oxidation,or loss of moisture within the samples through evaporation. The thermal behaviour of synthesized DESs was examined by using the TGA method; thermogravimetric data are illustrated in Table S1.
The thermal stabilities of IL mixtures with different HBDs can be quantified by measuring the decomposition temperature (Tdec),which denotes the temperature of initial 10% weight loss throughout the entire course of TGA scanning [34]. The manganese (II)-sugar developed DESs given as DES3,DES4 and DES5 have distinctly lower Tdec values (103 ℃,108 ℃,and 103 ℃,respectively). DES1 has the greatest thermal stability among the five DESs obtained,which can remain stable up to 193 ℃,followed by DES2 (stable up to 146 ℃). This study had shown that DESs with improved thermal stability could be obtained by mixing two original solid components; and this would be highly beneficial in the solvent industry. Heating of these manganese-based DESs would first leads to dehydration process,which involves the release of coordinated water molecules. Subsequently,multi-stage decomposition occurs with the degradation of DES involving the release of both available organic and chlorine parts. The final residue that was present in the nitrogen atmosphere towards the end of the heating process mainly is a manganese complex such as MnO [35].
3.3. Viscosity measurementViscosity study is one of the most important analyses because the application of DESs and ILs depends largely on individual viscosity. Since viscosity is largely affected by the presence of water,thus methanol instead of water was used in the evaporating method to ensure higher purity in DESs obtained. The lower the value of viscosity,the better the DES could be used as a solvent [24]. In general,DES with high viscosity is not advisable as it has its limitation in actual applications such as liquid-liquid extraction and electrochemical reactions [24, 36]. Viscosity of a DES largely affects the application temperature of the DES. Higher temperature could be applied to lower down the viscosity of a certain DES [15]; however,that would necessitate extra processing time,power and cost. The viscosity (η) of all synthesized manganese (II)-based DESs at room temperature (21 ℃) are presented in Table 1.
Viscosity reflects the interaction between molecules in a liquid mixture [27]. As refer to Table 1,DES1 with acetamide as HBD has the lowest viscosity value. The reason being that there is lesser intermolecular hydrogen bonding between the two original components that mixed. Whereas,DES2 has higher viscosity value compared to DES3 and DES4. This might be caused by the mole ratio of original components MnCl2-4H2O with glycerol (1:3),such mole ratio would resultant in higher polarity giving higher hydrogen bonding ability and thus less mobility within the free components [37]. Hence,such findings suggested that DES2,DES3 and DES4 might not be proper candidates for industrial DES applications.
On the other hand,DES5 has the highest viscosity among the five DESs as what expected owing to the extra 1 mole of D(-)- fructose. In majority of cases,despite of merely the presence of hydrogen bonding networks within DES5,this would suggests that there are more van der Waals and electrostatic interactions between the two components [27, 29]. This highlights the varieties among eutectic compositions,as they would possess different physical characteristics.
3.4. Conductivity in synthesized DESsIonic conductivity can be defined as the free ions within the material not trapped in the lattice sites but can escape to the adjacent site when given enough energy. Table 1 shows the ionic conductivity (σ) of synthesized manganese(II)-based DESs with different HBDs.
The conductivity of DES is highly affected by its viscosity and ion concentration [27]. Zhang and team stated that viscous DES would give low conductivity [29]. As hypothesized,the highest viscosity DES5 has the lowest conductivity value (Table 1). Conductivity is measured according to the mobility of ions through void volume within the binary DES liquid mixture. As refer to Table 1,DES1 that has the highest conductivity is believed to exhibit higher ion mobility with larger void volume build within the solution,followed by DES3 and DES4. This can be explained as the weak intermolecular hydrogen bond in DES1 that created larger free volume as compared to DES3 and DES4,due to the existence of more robust 3D intermolecular hydrogen-bond network with sugar components [29].
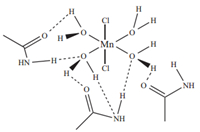
4. ConclusionTo sum up,five manganese(II)-based DESs were successfully synthesized in this study. It was shown that the physical properties of different DESs are highly dependent on the mole ratio of hydrated metal salt to HBD. DES1 (speculated chemical interactions can be found in Fig. 1) that exhibited the lowest freezing point,lowest viscosity,highest conductivity and highest thermal stability represents a promising candidate for chemical industrial applications. In-depth investigations such as Differential Scanning Colorimetry (DSC),X-crystallography and mass spectrometric tests shall be implemented in near future to provide further evidences to support current findings. Preliminary results obtained from this fundamental study are useful for related process scale-up studies as well as the development of metal-based DES applications in a wide range of chemical industrial applications.
|
Download:
|
| Fig. 1. Speculated chemical interactions within MnCl2-4H2O-acetamide (DES1). | |
Supplementary material related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.05.049.
| [1] | P. Wasserschield, T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2003. |
| [2] | M.A. Kareem, F.S. Mjalli, M.A. Hashim, I.M. Alnashef, Phosphonium-based ionic liquids analogues and their physical properties, J. Chem. Eng. Data 55(2010) 4632-4637. |
| [3] | J. Sun, M. Forsyth, D.R. MacFarlene, Room-temperature molten salts based on the quaternary ammonium ion, J. Phys. Chem. B 102(1998) 8858-8864. |
| [4] | S.J. Zhang, Y.H. Chen, R.X.F. Ren, et al., Solubility of CO2 in sulfonate ionic liquids at high pressure, J. Chem. Eng. Data 50(2005) 230-233. |
| [5] | A. Paiva, R. Craveiro, I. Aroso, et al., Natural deep eutectic solvents-solvents for the 21st century, ACS Sust. Chem. Eng. 2(2014) 1063-1071. |
| [6] | C.M. Wang, X.Y. Luo, X. Zhu, et al., The strategies for improving carbon dioxide chemisorption by functionalized ionic liquids, RSC Adv. 3(2013) 15518-15527. |
| [7] | M.A. Navarra, J. Manzi, L. Lombardo, S. Panero, B. Scrosati, Ionic liquid-based membranes as electrolytes for advanced lithium polymer batteries, Chem-SusChem 4(2011) 125-130. |
| [8] | X. Ge, C.D. Gu, Y. Lu, X.L. Wang, J.P. Tu, A Versatile protocol for the ionothermal synthesis of nanostructured nickel compounds as energy storage materials from a choline chloride-based ionic liquid, J. Mater. Chem. A 1(2013) 13454-13461. |
| [9] | M. Hayyan, F.S. Mjalli, M.A. Hashim, I.M. Alnashef, X.M. Tan, Electrochemical reduction of dioxygen in bis (trifluoromethylsulfonyl) imide based Ionic liquids, J. Electroanal. Chem. 657(2011) 150-157. |
| [10] | P.D. de María, Z. Maugeri, Ionic liquids in biotransformations:from proof-ofconcept to emerging deep-eutectic-solvents, Curr. Opin. Chem. Biol. 15(2011) 220-225. |
| [11] | N.V. Plechkova, K.R. Seddon, Applications of ionic liquids in the chemical industry, Chem. Soc. Rev. 37(2008) 123-150. |
| [12] | C. Ruß, B. König, Low melting mixtures in organic synthesis-an alternative to ionic liquids? Green Chem. 14(2012) 2969-2982. |
| [13] | X. Ge, C.D. Gu, X.L. Wang, J.P. Tu, Endowing manganese oxide with fast adsorption ability through controlling the manganese carbonate precursor assembled in ionic liquid, J. Colloid Interface Sci. 438(2015) 149-158. |
| [14] | D.V. Wagle, H. Zhao, G.A. Bakar, Deep eutectic solvents:sustainable media for nanoscale and functional materials, Acc. Chem. Res. 47(2014) 2299-2308. |
| [15] | M. Hayyan, F.S. Mjalli, M.A. Hashim, I.M. Alnashef, An investigation of the reaction between 1-butyl-3-methylimidazolium trifluoromethanesulfonate and superoxide ion, J. Mol. Liq. 181(2013) 44-50. |
| [16] | Y.S. Hu, Z.X. Wang, X.J. Huang, L.Q. Chen, Physical and electrochemical properties of new binary room-temperature molten salt electrolyte based on LiBETI and acetamide, Solid State Ionics 175(2004) 277-280. |
| [17] | E.R. Cooper, C.D. Andrews, P.S. Wheatly, et al., Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues, Nature 430(2004) 1012-1016. |
| [18] | M. Francisco, A. van den Bruinhorst, M.C. Kroon, Low-Temperature-Temperature Mixtures (LTTMs):a new generation of designer solvents, Angew. Chem. Int. Ed. 52(2013) 3074-3085. |
| [19] | A.P. Abbott, M. Azam, K.S. Ryder, S. Saleem, In situ electrochemical digital holographic microscopy; a study of metal electrodeposition in deep eutectic solvents, Anal. Chem. 85(2013) 6653-6660. |
| [20] | A.P. Abbott, K. El Ttaib, G. Frisch, K.J. McKenzie, K.S. Ryder, Electrodeposition of copper composites from deep eutectic solvents based on choline chloride, Phys. Chem. Chem. Phys. 11(2009) 4269-4277. |
| [21] | C.D. Gu, J.L. Zhang, W.Q. Bai, et al., Electro-brush plating from deep eutectic solvent:a case of nanocrystalline Ni coatings with superior mechanical property and corrosion resistance, J. Electrochem. Soc. 162(2015) D159-D165. |
| [22] | G.F. Cai, J.P. Tu, C.D. Gu, et al., One-step fabrication of nanostructured NiO films from deep eutectic solvent with enhanced electrochromic performance, J. Mater. Chem. A 1(2013) 4286-4292. |
| [23] | C.D. Gu, J.P. Tu, One-step fabrication of nanostructured Ni film with lotus effect from deep eutectic solvent, Langmuir 27(2011) 10132-10140. |
| [24] | Y.T. Dai, J. van Spronsen, G.J. Witkamp, R. Verpoorte, Y.H. Choi, Natural deep eutectic solvents as new potential media for green technology, Anal. Chim. Acta 766(2013) 61-68. |
| [25] | J.C. Barron, The Electrochemistry of Zn in Deep Eutectic Solvents,Ph.D thesis, University of Leicester, 2009. |
| [26] | A.P. Abbott, G. Capper, D.L. Davies, R.K. Rasheed, V. Tambyrajah, Ionic liquids and their use as solvent, US Patent, 7183433 B2,2007. |
| [27] | W.J. Guo, Y.C. Hou, S.H. Ren, S.D. Tian, W.Z. Wu, Formation of deep eutectic solvents by phenols and choline chloride and their physical properties, J. Chem. Eng. Data 58(2013) 866-872. |
| [28] | M. Avalos, R. Babiano, P. Cintas, J.L. Jiménez, J.C. Palacios, Greener media in chemical synthesis and processing, Angew. Chem. Int. Ed. 45(2006) 3904-3908. |
| [29] | Q.H. Zhang, K. De Oliveira Vigier, S. Royer, F. Jérôme, Deep eutectic solvents:syntheses, properties and applications, Chem. Soc. Rev. 41(2012) 7108-7146. |
| [30] | H.Y. Wang, Y. Jing, X.H. Wang, Y. Yao, Y.Z. Jia, Structure and physico-chemical properties of three analogous ionic liquids containing magnesium chloride, J. Mol. Liq. 170(2012) 20-24. |
| [31] | W.Y. Guo, X.M. Zhang, Metal-ion interactions with sugars. The crystal structure and FTIR study of an SrCl2-fructose complex, Carbohydr. Res. 339(2004) 1421-1426. |
| [32] | K. Dill, S. Bromberg, Molecular Driving Forces:Statistical Thermodynamics in Biology, Chemistry, Physics, and Nanoscience, Garland Science, San Diego, 2010, pp. 616-617. |
| [33] | G. Aullón, D. Bellamy, A.G. Orpen, L. Brammer, E.A. Bruton, Metal-bound chlorine often accepts hydrogen bonds, Chem. Commun. 6(1998) 653-654. |
| [34] | H. Zhao, G.A. Baker, S. Holmes, New eutectic ionic liquids for lipase activation and enzymatic preparation of biodiesel, Org. Biomol. Chem. 9(2011) 1908-1916. |
| [35] | R. Mrozek, Z. Rzaçzyńska, M. Sikorska-Iwan, Thermal analysis of manganese (II) complexes with glycine, J. Therm. Anal. Calorim. 63(2001) 839-846. |
| [36] | A.A. Shamsuri, D.K. Abdullah, Ionic liquids:preparations and limitations, Makara Sains 14(2010) 101-106. |
| [37] | J.I. García, H. García-Martín, E. Pires, Glycerol based solvents:synthesis, properties and applications, Green Chem. 16(2014) 1007-1033. |
 2015, Vol.26
2015, Vol.26