b CNOOC Tianjin Chemical Research and Design Institute, Tianjin 300131, China
Conjugated polyenes have been widely applied as molecular rods,organic electro-optic materials,light-emitting diodes,electrochromic systems,liquid crystal materials,and anticoccidial agents [1, 2, 3, 4, 5]. As members of this family,1,4-diarylbutadienes exhibit interesting reversible photochemical phase transition behavior [4] and moderate bioactivity [5]. Introducing alkyl substituents onto the conjugated backbone provides a good way to improve the solubility and stability of these compounds. Therefore,it is important to develop methods to prepare alkylsubstituted short polyenes.
However,there are few reports on the preparation of 1,2,3,4- tetraalkyl-1,4-diarylbutadienes due to some challenging obstacles. Gage et al. [5] synthesized 1,4-diarylbutadienes in low yields. Jiang et al. [6] reported Pd-catalyzed three-component coupling of aryl iodides,internal alkynes,and arylboronic acid to provide 1,2,3,4- tetrapropyl-1,4-diarylbutadienes in the harsh condition of supercritical carbon dioxide. Larock et al. [7] covered the formation of 1,2,3,4-tetrapropyl-1,4-diarylbutadienes with poor selectivity. Xi et al. [8] prepared 1,2,3,4-tetraethyl-1,4-diarylbutadienes as a side product.
In 1986,Negishi et al. [9] proposed a convenient way to treat Cl2ZrCp2 with alkylmetals to produce organozirconium species that act as sources of ‘‘ZrCp2’’,the latter product being a convenient reagent for preparing zirconacycles,which were proved to be useful building blocks for the formation of C-C bonds. On the basis of previous work,Takahashi et al. carried out the reactions of zirconacyclopentadienes with aryl iodides or alkynyl iodides [10],allyl chlorides [11],and alkynyl halides [12] in the presence of CuCl,which offered mono-arylated-1,3-butadienes,tetraenes,and 1-alkynyl-1,3-butadienes or 1,4-dialkynyl-1,3-butadienes along with the formation of one sp2C-sp2C bond,two sp2C-sp3C bonds and one or two sp2C-spC bonds,respectively. Even so,the formation of two sp2C-sp2C bonds in these reactions did not occur at all,indicating that it is very difficult to form two sp2C-sp2C bonds in one-pot reactions and there is a limitation when using CuCl only. So,it is necessary to activate the substrate to promote such reaction.
According to the literature,palladium catalyst is efficient in coupling reactions. Larock et al. [13] proposed Pd-catalyzed addition of arylboronic acids to internal alkynes to provide a wide variety of tetrasubstituted olefins. Satoh et al. [14] demonstrated that the coupling reactions of aryl boronic acids with alkynes can be performed with palladium catalyst to give multiarylated butadienes. Miura et al. [15] optimized the process to produce 1,4-diaryl-1,3-butadienes by adding triaryl phosphate,but the product yields were still moderate due to the facile deactivation of the palladium catalyst under the oxidative conditions.
Based on the above,we propose that the combination of CuCl and palladium catalyst may promote the formation of two sp2C- sp2C bonds which will offer bis-arylated-1,3-butadienes. Combined with the zirconocene-mediated reactions we reported earlier [16],it is assumed that after the cleavage of Zr-C bonds of zirconacyclopentadienes,two sp2C-sp2C bonds may be formed,catalyzed by palladium along with arylation.
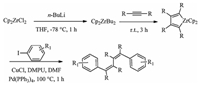
In this letter,we would like to report the first efficient and selective formation of two sp2C-sp2C bonds in one-pot reaction via the palladium-catalyzed cross-coupling of zirconacyclopentadienes with aryl iodides to afford 1,2,3,4-tetraalkyl-1,4-diarylbutadienes (Scheme 1).
|
Download:
|
| Scheme. 1.Synthesis of 1,2,3,4-tetraalkyl-1,4-diarylbutadienes via palladium-catalyzed coupling from zirconacyclopentadienes with aryl iodides. | |
All reactions including air- and moisture-sensitive materials were carried out with standard Schlenk techniques under nitrogen atmosphere. Tetrahydrofuran (THF) was distilled over sodium benzophenone ketyl under a positive pressure of dry nitrogen. 1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone(DMPU,ALDRICH,98%) was dried over calcium hydride and distilled under reduced pressure. All the other reagents were commercially available (TCI,ALDRICH,KANTO,WAKO) and used without further purification. 1H NMR and 13C NMR spectra were recorded in CDCl3 solutions on JEOL JNM-AL300 NMR spectrometers (FT,300 MHz for 1H; 75 MHz for 13C) at room temperature unless otherwise noted. GC analyses were performed on SHIMADZU GC-14B.
Typical procedure for the preparation of 1,2,3,4-tetraalkyl-1,4- diarylbutadienes (2a-j): To a solution of Cp2ZrCl2 (350.8 mg,1.2 mmol) in anhydrous THF (5 mL) was added n-BuLi (1.64 mol/L hexane solution,1.46 mL,2.4 mmol) drop-wise via syringe under nitrogen atmosphere at -78 ℃. The mixture was vigorously stirred for 1 h,then alkyne (2 mmol) was added to the solution,and the reaction mixture was gradually warmed to room temperature by removal from the cooling bath. After stirring for 3 h,the mixture was cooled to 0 ℃,CuCl (238.8 mg,2.4 mmol),Pd(PPh3)4 (57.8 mg,0.05 mmol,5 mol%),aryl iodides (4 mmol),DMF (5 mL) and tetrahydro-1,3-dimethyl-2(1H)pyrimidine (DMPU,0.60 mL) were sequentially added to the mixture. After stirring for 1 h at 100 ℃ under nitrogen atmosphere,the reaction was quenched with 3 mol/L HCl and extracted with ethyl acetate. The combined organic phase was sequentially washed with water,saturated aqueous NaHCO3 solution,and brine. Then the solution was dried over anhydrous MgSO4. The organic solvent was evaporated by a rotary evaporator,and the residue was purified by column chromatography on silica gel (pure n-hexane as eluent) to afford the corresponding products.
3,6-Diphenyl-4,5-diethylocta-3,5-diene (2a): GC yields 96% (isolated yield 78%). 1H NMR (CDCl3,300 MHz): δ 0.81 (t,6H,J = 7.7 Hz),0.97 (t,6H,J = 7.4 Hz),1.64-1.78 (m,4H),2.11-2.42 (m,4H),7.07-7.23 (m,10H); 13C NMR (CDCl3,75 MHz): δ 12.77,13.96,26.50,26.81,125.64,127.33,128.63,137.51,139.51,143.64. HRMS calcd. for C24H30 318.2348,found 318.2344.
4,7-Diphenyl-5,6-dipropyldeca-4,6-diene (2b): GC yields 82% (isolated yield 49%). 1H NMR (CDCl3,300 MHz): δ 0.80-0.94 (m,12H),1.18-1.56 (m,8H),1.62-1.76 (m,2H),2.06-2.20 (m,2H),2.22-2.40 (m,4H),7.13-7.33 (m,10H); 13C NMR (CDCl3,75 MHz): d 14.13,14.83,21.44,22.70,36.04,36.43,125.63,127.36,128.66,136.70,139.22,143.91. HRMS calcd. for C28H38 374.2974,found 374.2975.
5,8-Diphenyl-6,7-dibutyldodeca-5,7-diene (2c): GC yields 68% (isolated yield 42%). 1H NMR(CDCl3,300 MHz): δ 0.89 (t,6H,J = 6.8 Hz),0.96 (t,6H,J = 7.2 Hz),1.24-1.53 (m,16H),1.68-1.80 (m,2H),2.11-2.24 (m,2H),2.28-2.44 (m,4H),7.17-7.32 (m,10H); 13C NMR (CDCl3,75 MHz): δ 13.94,13.95,22.74,23.36,30.45,31.61,33.69,33.84,125.60,127.32,128.63,136.65,139.10,143.99. HRMS calcd. for C32H46 430.3600,found 430.3603.
3,6-Di(2-methyl)phenyl-4,5-diethylocta-3,5-diene (2d): GC yields 74% (isolated yield 44%). 1H NMR(CDCl3,300 MHz): δ 0.75 (t,6H,J = 7.2 Hz),0.89 (t,6H,J = 7.3 Hz),1.20-1.36 (m,2H),1.72- 1.99 (m,4H),2.23 (s,6H),2.41-2.56 (m,2H),7.11-7.38 (m,8H); 13C NMR (CDCl3,75 MHz): δ 12.50,14.27,19.51,24.58,26.51,124.97,126.17,129.43,129.90,135.62,137.50,140.22,143.15. HRMS calcd. for C26H34 346.2661,found 346.2659.
3,6-Di(3-methyl)phenyl-4,5-diethylocta-3,5-diene (2e): GC yields 98% (isolated yield 63%). 1H NMR(CDCl3,300 MHz): δ 0.87 (t,6H,J = 7.4 Hz),1.00 (t,J = 7.5 Hz,6H),1.66-1.80 (m,2H),2.15- 2.48 (m,6H),2.34 (s,6H),6.98-7.07 (m,6H),7.12-7.19 (m,2H); 13C NMR (CDCl3,75 MHz): δ 12.80,13.99,21.47,26.49,26.66,125.69,126.30,127.18,129.34,136.56,137.29,139.52,143.59. HRMS calcd. for C26H34 346.2661,found 346.2660.
3,6-Di(4-methyl)phenyl-4,5-diethylocta-3,5-diene (2f): GC yields 82% (isolated yield 51%). 1H NMR(CDCl3,300 MHz): δ 0.85 (t,6H,J = 7.3 Hz),0.94 (t,6H,J = 7.4 Hz),1.54-1.69 (m,2H),2.08- 2.50 (m,6H),2.36 (s,6H),7.01-7.09 (m,4H),7.12-7.19 (m,4H); 13C NMR (CDCl3,75 MHz): δ 12.68,13.93,21.13,26.54,26.79,128.15,128.44,135.15,136.93,139.57,140.67. HRMS calcd. for C26H34 346.2661,found 346.2658.
3,6-Di(4-methoxy)phenyl-4,5-diethylocta-3,5-diene (2g): GC yields 81% (isolated yield 70%). 1H NMR(CDCl3,300 MHz): δ 0.83 (t,6H,J = 7.7 Hz),0.94 (t,6H,J = 7.5 Hz),1.59-1.72 (m,2H),2.09- 2.42 (m,6H),3.79 (s,6H),6.73-6.80 (m,4H),7.07-7.15 (m,4H); 13C NMR (CDCl3,75 MHz): δ 12.79,13.96,26.41,26.75,55.14,112.74,129.57,136.05,136.68,139.24,157.59. HRMS calcd. for C26H34O2 378.2559,found 378.2800.
3,6-Di(4-bromo)phenyl-4,5-diethylocta-3,5-diene (2h): GC yields 56% (isolated yield 24%). 1H NMR(CDCl3,300 MHz): δ 0.76 (t,6H,J = 7.5 Hz),1.04 (t,6H,J = 7.5 Hz),1.76-1.96 (m,2H),2.07- 2.37 (m,6H),6.74-6.82 (m,4H),7.23-7.31 (m,4H); 13C NMR (CDCl3,75 MHz): δ 12.85,13.99,26.50,26.53,119.44,130.32,130.38,137.09,139.55,142.23. HRMS calcd. for C24H28Br2 474.0558,found 474.0561.
3,6-Di(benzoic acid ethyl ester)-4,5-diethylocta-3,5-diene(2i): GC yields 76% (isolated yield 55%). 1H NMR (CDCl3,300 MHz): δ 0.72 (t,6H,J = 7.4 Hz),1.06 (t,6H,J = 7.5 Hz),1.38 (t,6H,J = 7.2 Hz),1.83-1.98 (m,2H),2.09-2.36 (m,6H),4.36 (q,4H,J = 7.2 Hz),6.89- 6.96 (m,4H),7.79-7.86 (m,4H); 13C NMR (CDCl3,75 MHz): δ 12.72,13.94,14.25,26.38,26.59,60.63,127.62,128.47,128.54,137.76,140.11,148.27,166.62. HRMS calcd. for C30H38O4 462.2770,found 462.2772.
3-(4-Nitro)phenyl-4,5-diethylocta-3,5-diene (3): GC yields 60% (isolated yield 31%). 1H NMR (CDCl3,300 MHz): δ 0.60 (t,3H,J = 7.5 Hz),0.80-0.95 (m,6H),1.00 (t,3H,J = 7.5 Hz),1.71-1.86 (m,2H),1.93 (q,2H,J = 7.5 Hz),2.27 (q,2H,J = 7.5 Hz),2.43 (q,2H,J = 7.5 Hz),4.85 (t,1H,J = 7.3 Hz),7.89-7.97 (m,2H),8.02-8.11 (m,2H); 13C NMR (CDCl3,75 MHz): δ 13.18,13.23,13.32,13.66,20.96,22.77,24.19,26.80,122.65,124.83,130.27,133.77,135.71,138.66,139.11,152.18. HRMS calcd. for C18H25NO2 287.1885,found 287.1887.
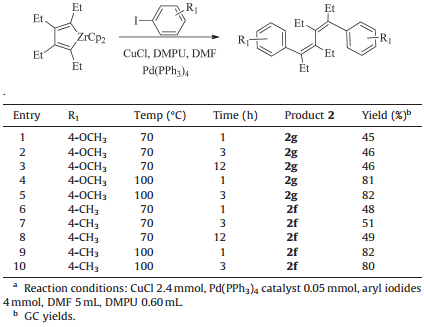
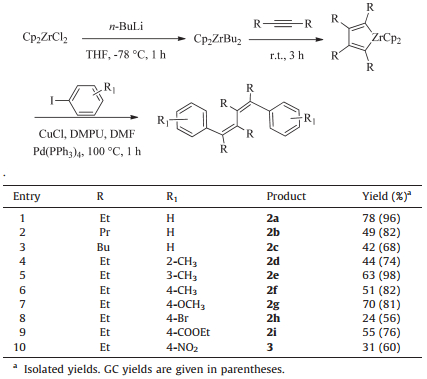
3. Results and discussionIn this letter,we report a novel and effective procedure for the one-pot synthesis of 1,2,3,4-tetraalkyl-1,4-diarylbutadienes from two internal alkynes and aryl iodides. Our research started with the preparation of zirconacyclopentadiene. Alkyne was added to a solution of Cp2Zr(n-Bu)2 (Negishi reagent) prepared from zirconocene dichloride with two equiv. of n-BuuLi in THF at -78 ℃ under nitrogen. The reaction mixture was gradually warmed to room temperature and stirred for 1 h to give zirconacyclopentadiene in high yield [10]. Then,we set out to develop conditions for this transformation employing palladium catalyst and zirconacyclopentadienes as intermediates in this reaction. The model reaction was carried out in the presence of Pd(PPh3)4 catalyst with an amount of 5 mol% which could efficiently promote the formation of one sp2C-sp2C bond between alkenylzirconocenes and aryl iodides [17].
In order to optimize the conditions,4-iodoanisole and 4- iodotoluene were chosen as models in the presence of palladium catalyst and CuCl under different conditions. Initially,the reaction of 4-iodoanisole with zirconacyclopentadiene was carried out at 70 ℃ for 1 h,and the product 2 could be obtained in a GC yield of 45%. After stirring for another 2 h,monitored by GC,even though 92% of zirconacyclopentadiene was consumed,only 46% GC yield of product 2 was obtained. When prolonging the reaction time to 12 h,it had no effect on the GC yield (46%). When performing the reaction at 100 ℃ for 1 h,a GC yield of 81% was obtained. After continuing the reaction for another 2 h,no significant differences appeared. The reaction conditions also worked well for 4- iodotoluene. When using 4-iodotoluene as substrate and controlling the reaction at 100 ℃ for 1 h,the GC showed a yield of 82%. Therefore,the optimized reaction condition was 100 ℃ for 1 h (Table 1).
| Table 1 Optimization of reaction conditions.a |
With the optimized conditions in hand,the scope of this method with a series of aryl iodides was investigated and the results are shown in Table 2. These results showed that this reaction tolerated a variety of aryl iodides,including electronwithdrawing groups as well as electron-donating groups on the benzene ring to produce the desired compounds (2a-i) with moderate to excellent yields. It is also concluded that: (1) The yields of 1,2,3,4-tetraalkyl-1,4-diarylbutadienes decreased dramatically from 96% to 68% in the case of 2a-2c due to using larger R groups (from ethyl to butyl) in the zirconacyclopentadienes. (2) When the substituent was in the ortho position of iodine in aryl iodide,it seemed that the yield is lower than that in the meta or para position because of the steric effects as shown in 2e and 2f. (3) The results of 2g-2i showed that electron-withdrawing groups in the para position of iodine affected the reaction more adversely than electron-donating groups. In addition,only mono-coupling product was found when nitro-group was in the para position,as in the case of 3 (entry 10).
| Table 2 Scope of the substrates for the preparation of 1,2,3,4-tetraalkyl-1,4-diarylbutadienes. |
Mechanistically,the Pd-catalyzed coupling reaction was proposed as an oxidation addition-reductive elimination process (Scheme 2). When 2.4 equiv. CuCl was added to the system,the reaction with 1 equiv. zirconacyclopentadiene afforded Cl2ZrCp2 and organocopper intermediate 4. It had been proved that 4 could not proceed in the coupling reaction with aryl iodide directly [18]. However,the transmetalation process achieved a combination of aryl group and butadiene group by Pd(II) specie. The product 2 was obtained after the reductive elimination process and the Pd(II) was reduced back to Pd(0) catalyst.

|
Download:
|
| Scheme. 2.Proposed reaction mechanism with Cu(I) and Pd(0). | |
In conclusion,we disclosed a novel and efficient approach for the preparation of 1,2,3,4-tetraalkyl-1,4-diarylbutadienes from two molecule internal alkynes and aryl iodides. In this protocol,zirconium promoted the transformation of alkynes into zirconacyclo pentadienes. Then,mediated by cuprous chloride and catalyzed by palladium catalyst,the coupling reaction of zirconacyclopentadienes with aryl iodides occurred via the formation of two sp2C-sp2C bonds. The desired products can be generated selectively in moderate to excellent yields in one-pot reaction. This reaction showed a broad substrate scope and a wide variety of functional groups were compatible. Meanwhile,this method extends the application of zirconacyclopentadienes and offers a straightforward route to prepare multiarylated butadienes,which are of interest for their photochemical,electrochemical,and biological properties.
AcknowledgmentWe are grateful for Professor Tamotsu Takahashi in Hokkaido University for his kind suggestions and help.
| [1] | (a) P.F.H. Schwab, J.R. Smith, J. Michl, Synthesis and properties of molecular rods. 2. Zig-Zag rods, Chem. Rev. 105(2005) 1197-1279;(b) H. Meier, Conjugated oligomers with terminal donor-acceptor substitution, Angew. Chem. Int. Ed. 44(2005) 2482-2506;(c) L.R. Dalton, P.A. Sullivan, D.H. Bale, Electric field poled organic electro-optic materials:state of the art and future prospects, Chem. Rev. 110(2010) 25-55. |
| [2] | J.H. Kim, S. Noh, K. Kim, et al., Blue light emitting diode with1,1,4,4-tetraphenyl-1,3-butadiene (TPB), Synth. Met. 117(2001) 227-228. |
| [3] | T. Suzuki, H. Higuchi, M. Ohkita, et al., Dual-mode electrochromism switched by proton transfer:dynamic redox properties of bis(diarylmethylenium)-type dyes, Chem. Commun. (2001) 1574-1575. |
| [4] | R. Davis, V.A. Mallia, S. Das, Reversible photochemical phase transition behavior of alkoxy-cyano-substituted diphenylbutadiene liquid crystals, Chem. Mater. 15(2003) 1057-1063. |
| [5] | J.L. Gage, H.A. Kirst, D. ONeil, et al., Synthesis and evaluation of a series of 1,4-diarylbutadienes for anticoccidial activity, Bioorg. Med. Chem. 11(2003) 4083-4091. |
| [6] | H.F. Jiang, Q.X. Xu, A.Z. Wang, Stereoselective synthesis of tetrasubstituted olefins via palladium-catalyzed three-component coupling of aryl iodides, internal alkynes, and arylboronic acids in supercritical carbon dioxide, J. Supercrit. Fluids 49(2009) 377-384. |
| [7] | C.X. Zhou, R.C. Larock, Regio and stereoselective route to tetrasubstituted olefins by the palladium-catalyzed three-component coupling of aryl iodides, internal alkynes, and arylboronic acids, J. Org. Chem. 70(2005) 3765-3777. |
| [8] | G.T. Li, H.Y. Fang, S.W. Zhang, et al., Synthesis, structural characterization, and skeletal rearrangement of dibenzo tricyclo[3.3.0.02,6]-1,2,5,6-tetrasubstituted octanes, Tetrahedron Lett. 45(2004) 8399-8402. |
| [9] | E. Negishi, F.E. Cederbaum, T. Takahashi, Metal-promoted cyclization. 11. Reaction of zirconocene dichloride with alkyllithiums or alkyl Grignard reagents as a convenient method for generating a zirconocene equivalent and its use in zirconium-promoted cyclization of alkenes, alkynes, dienes, enynes, and diynes, Tetrahedron Lett. 27(1986) 2829-2832. |
| [10] | T. Takahashi, W.H. Sun, C.J. Xi, et al., Selective one carbon-carbon bond formation reaction of zirconacyclopentadienes with aryl iodides or alkynyl iodides, Tetrahedron 54(1998) 715-726. |
| [11] | T. Takahashi, M. Kotoral, K. Kasai, et al., Novel syntheses of eight-membered-fivemembered fused-ring compounds from zirconacyclopentadienes, Organometallics 13(1994) 4183-4185. |
| [12] | R. Hara, Y.H. Liu, W.H. Sun, et al., Highly substituted enyne formation by coupling reaction of alkenylzirconium compounds with alkynyl halides, Tetrahedron Lett. 38(1997) 4103-4106. |
| [13] | C.X. Zhou, R.C. Larock, Tetrasubstituted olefin synthesis via Pd-catalyzed addition of arylboronic acids to internal alkynes using O2 as an oxidant, J. Org. Chem. 71(2006) 3184-3191. |
| [14] | T. Satoh, S. Ogino, M. Miura, et al., Synthesis of highly substituted 1,3-butadienes by palladium-catalyzed arylation of internal alkynes, Angew. Chem. Int. Ed. 43(2004) 5063-5065. |
| [15] | H. Horiguchi, H. Tsurugi, T. Satoh, et al., Palladium/phosphite or phosphate catalyzed oxidative coupling of arylboronic acids with alkynes to produce 1,4-diaryl-1,3-butadienes, Adv. Synth. Catal. 350(2008) 509-514. |
| [16] | (a) H.M. Qu, X.H. Niu, J. Li, et al., Synthesis and structure determination of novel hexasubstituted cyclohexadienes, Chin. Chem. Lett. 23(2012) 1137-1140;(b) S. Li, H.M. Qu, L.S. Zhou, et al., Zircomium-mediated selective synthesis of 1,2,4,5-tetrasubstituted benzenes from two sily-substituted alkynes and one internal alkyne, Org. Lett. 11(2009) 3318-3321;(c) T. Seri, H.M. Qu, L.S. Zhou, et al., Substituent effects in the preparation of naphthacenes by the coupling reaction of diyne-derived zirconacyclopentadienes with tetraiodobenzene, Chem. Asian J. 3(2008) 388-392;(d) H.M. Qu, J. Zhang, J. Li, et al., Synthesis of hexa-substituted benzenes with trimethylsilyl groups mediated by Negishi reagent and Cu or Ni compound, Tianjin Daxue Xuebao 45(2012) 770-774;(e) J.K. Tang, X.H. Niu, L.L. Jiang, et al., Crystal structure, photoluminescence and theoretical studies of diethyl 4,5-di(thienyl)-3,6-bis (trimethylsilyl)phthalate, Chin. J. Struct. Chem. 32(2013) 1560-1566. |
| [17] | R. Hara, Y. Nishihara, P.D. Landré, et al., Coupling reaction of alkenylzirconocenes with aryl or alkenyl iodides in the presence of CuCl/Pd(PPh3)4, Tetrahedron Lett. 38(1997) 447-450. |
| [18] | L.S. Zhou, K. Nakajima, K.I. Kanno, et al., Synthesis of acenes via coupling of 1,4-dilithiobutadienes with diiodoarenes in the presence of CuCl, Tetrahedron Lett. 50(2009) 2722-2726. |
 2015, Vol.26
2015, Vol.26