Multicomponent reactions (MCRs),an important subclass of tandem reactions,are one-pot processes in which three or four easily approachable components react to form a single product. The methodology has emerged as a powerful synthetic tool for the preparation of biologically active compounds and important drugs [1, 2]. The multicomponent reactions have been used frequently in organic synthesis,and significant attempts have been focused on the design and development of environmentally friendly and less expensive methods for the generation of libraries of heterocyclic compounds [3, 4]. Therefore,academic and industrial research groups have increasingly focused on the development of MCRs that can lead to new,efficient,synthetic methodologies to afford several biologically-active compounds.
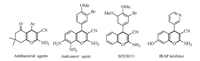
There has been considerable attention in syntheses,reactions and biological activities of 4H-pyran-containing molecules. Furthermore,4H-pyran derivatives also constitute a structural unit of some pharmaceutical agents,and natural products [5, 6]. The 2-amino-3-cyano-4H-pyran derivatives represent a significant class of compounds,viz. used in cosmetics and pigments,and utilized as potentially biodegradable agrochemicals [7]. Additionally, several poly functionalized 4H-pyran derivatives have been reported to show a variety of biological activities such as antitumor [8],antibacterial [9] and antimicrobial activities [10]. These compounds are structurally similar to the anticancer agent MX58151 and inhibitors of insulin-regulated amino peptidase (IRAP) related to enhancement of memory and learning functions [11] (Fig. 1). The 4H-pyran derivatives are also used as photoactive materials [12] and as synthetic intermediates for dihydrofurans [13].
|
Download:
|
| Fig. 1.2-Amino-3-cyano-4H-pyrans containing heterocycles demonstrating pharmacological and biological activity. | |
During the last several years,the diverse applications of such 2-amino-3-cyanopyran heterocyclic scaffolds in medicinal chemistry have drawn appreciable attention among synthetic chemists to explore useful synthetic routes to these heterocycles of potential interest. Therefore,numbers of good methods were reported, amongst them,the more interesting and impressive involves the synthesis of 2-amino-3-cyano-4H-pyran [14, 15, 16, 17] and spirooxindoles [18]. Owing to their medicinal utility,some reports on the multicomponent entries to 2-amino-3-cyano-4H-pyran and spirooxindoles appeared employing various catalysts [19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29]. Although these reported protocols find certain merits of their own,they still suffered from a number of disadvantages,such as longer reaction time,harsh reaction condition,heating,and tediouswork-up procedures. Thus,the development of more simple,clean, rapid and efficient methods to this important class of heterocycles is still needed.
Cesium fluoride (CsF) has emerged as an efficient,mild and basic catalyst used as a source of the fluoride ion in organic chemistry [30]. The synthetic approaches using CsF are found in rapid and efficient Knoevenagel-Michael condensation reactions [31] since the high affinity of CsF makes it capable of binding with the aldehyde carbonyl oxygen,thus increasing the reactivity of the parent carbonyl compounds.
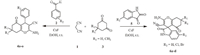
In continuation of our work on heterocycles synthesis [32], herein we envisioned the highly efficient and practical reactions of malononitrile,aromatic aldehydes or isatins and 5,5-dimethyl-1,3- cyclohexanedione or 1,3 cyclohexanedione in the presence of cesium fluoride in ethanol at room temperature to produce substituted 2-amino-3-cyano-4H-pyran and spirooxindole derivatives in quantitative yield (Scheme 1).
|
Download:
|
| Scheme. 1.CsF-catalyzed syntheses of 2-amino-3-cyano-4H-pyran and spirooxindole derivative. | |
Melting points were measured in open capillary tubes and uncorrected. FT-IR spectra were obtained on Shimadzu IR-Affinity spectrometer (KBr pellets). The 1H NMR were recorded on an NMR spectrometer,model Advance-II (Bruker). The instrument is equipped with a cryomagnet of field strength 9.4 T. Its 1H frequency is 400 MHz,using TMS as an internal standard and DMSO as a solvent. Chemical shifts are given in parts per million (d) and the coupling constants are given in Hertz. Silica gel-G plates (Merck) were used for TLC analysis with a mixture of acetone in n-hexane (15-30%) as the eluent. CsFwas obtained fromSpectrochem- Indian Company and was used without further purification.
General procedure for the preparation of 2-amino-3-cyano-4Hpyran and spirooxindole derivatives (4a-o and 6a-d) by a threecomponent system with CsF: A mixture of aromatic aldehydes or isatins (2 mmol),malononitrile (2 mmol),dimedone or 1,3- cyclohexanedione (2 mmol),and a catalytic amount of CsF (10 mol%) in ethanol was stirred at ambient temperature. The progress of the reaction was monitored by thin-layer chromatography (TLC). During the reaction,solid observed in flask within 2-5 min and after complete conversion,the reaction mass was transferred to an ice-water mixture under vigorous stirring. After stirring at room temperature,the solid was collected by filtration, washed with cold aq. ethanol,and dried. The authenticity of products was established by comparing their melting points with those reported in the literature and by the spectral data of FT-IR,1H NMR and mass analysis.
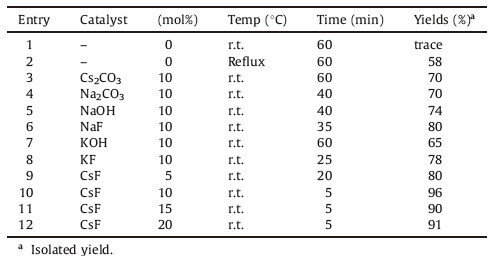
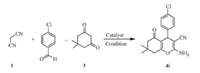
3. Results and discussionProspectively,to examine the catalytic role of CsF in ethanol, the reaction conditions were surveyed using malononitrile, p-chlorobenzaldehyde and dimedone (molar ratio 1:1:1) as the model reaction. (Scheme 2). To prove the efficiency of CsF,initially the reaction was carried out in the absence of catalyst,the anticipated product 4i (Table 1,entries 1 and 2) was limited to poor yields at r.t. and 58% yield at reflux with mainly unreacted 4-chlorobenzylidenemalononitrile realized. The reaction between dimedone and 4-chloro benzylidenemalononitrile requires catalyst, since without catalyst the reaction does not lead to the formation of the desired product. In order to confirm the effective involvement of CsF in the reaction,we carried out the model reaction with various metal salts including Na2CO3,NaOH,KOH, Cs2CO3,NaF and KF (Table 1,entries 3-6). In all cases,the reaction proceeded to completion and the results with other metal salts were unimpressive. However; the addition of a catalytic amount of CsF resulted in the formation of product 4i with 95% yield in 5 min at ambient temperature. This clearly indicates that the catalytic effect of CsF on the synthesis of 4i and based on these results,we then investigated the catalytic amount required for the reaction,and found that 10 mol% of CsF (Table 1,entry 4) catalyst gives excellent yield. Excess amounts of the catalyst did not improve the yield to a greater extent (Table 1,entries 5 and 6). Thus,a 10 mol% CsF was chosen as the maximum quantity of catalyst used for these reactions.
|
Download:
|
| Scheme. 2.Model reaction for 4i. | |
| Table 1Optimization of reaction conditions for the synthesis of 2-amino-3-cyano-4H-pyran (4i) derivatives using CsF. |
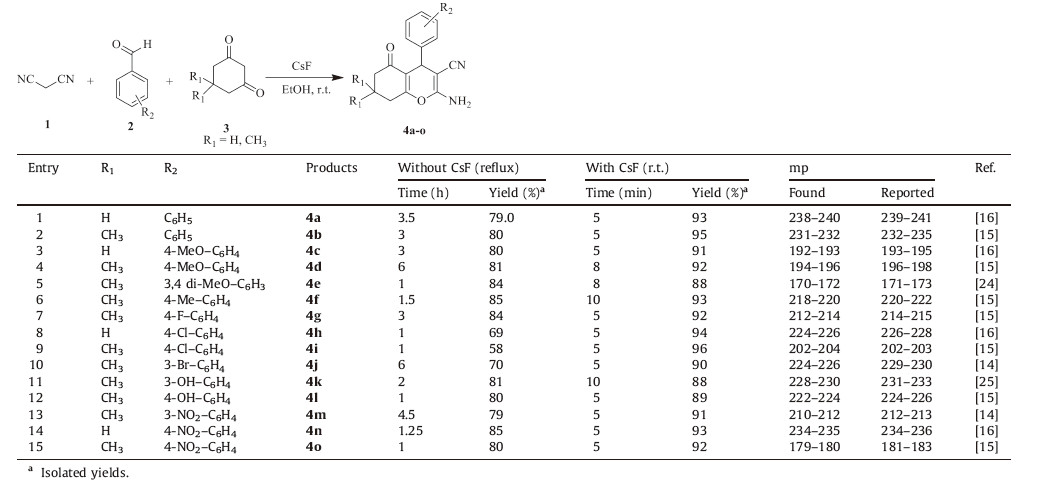
We explored the scope of this reaction and synthesized a series of 2-amino-3-cyano-4H-pyran derivatives (4a-o) by using a variety of aromatic aldehydes. In this context,the effects of CsF in the course of the reaction were initially examined on all reactions without CsF in ethanol at reflux conditions and found all reactions require 1-6 h to produce moderated yields. Therefore, using the optimized reaction conditions,the aldehydes having both the electron withdrawing (e.g. -4NO2,-3NO2) and electron donating substituents (e.g. -Me,-Cl,-OH,-OMe) participated in the reaction without any problem (Table 2). All the reactions shown here have very short reaction time (5-10 min) and high yields (88-96%). All the products were recovered from the reaction mixture and recrystallized from hot ethanol and dried thoroughly in an oven. The formation of products was initially confirmed by their melting point determination. All the 2-amino-3-cyano-4Hpyran derivatives synthesized are known compounds and we observed that the melting point of each 4H-pyran derivative was in good accordance with the literature values. The products were further confirmed by their spectroscopic data (FT-IR,1H NMR and Mass data).
| Table 2CsF mediated rapid condensation of 1,3-cyclohexadione with aromatic aldehydes.a |
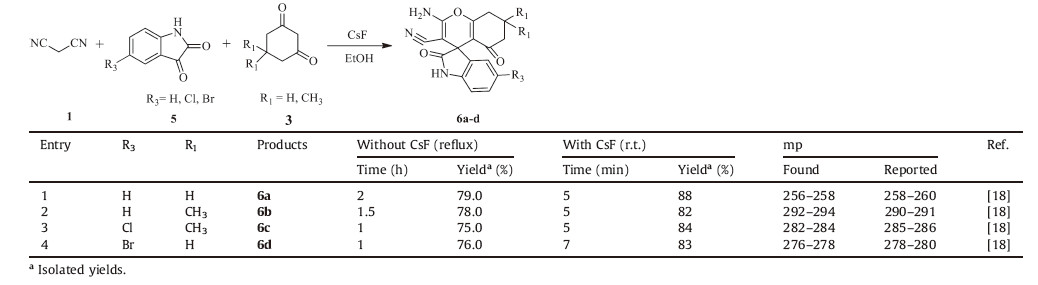
Encouraged by the above initial success and to further expand the scope of the present method,we turned to a one-pot synthesis of spirooxindoles by involving isatins,1,3 cyclohexanedione and malononitrile. In fulfillment of our expectations,under the aboveoptimized conditions,the reactions proceeded smoothly,and a variety of the desired spirooxindole products 6a-d was obtained in quantitative yields (Table 3).
| Table 3CsF mediated rapid condensation of spirooxindole derivatives. |
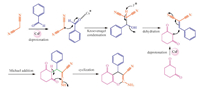
Herein,a reasonable mechanism for the formation of the product via tandem Knoevenagel cyclocondensation is outlined in Scheme 3. The function of cesium fluoride in this reaction is very important due to various reasons. It is well acknowledged that among alkaline metal salts,cesium salts have the lowest degree of solvation and ion pairing power which results in the cesium ion more accessible to coordinate more efficaciously with the carbonyl oxygen and thus,making the carbonyl carbon more electrophilic [33]. Initially,the cationic part of CsF coordinates with carbonyl oxygen of the arylaldehyde. Then the formation of arylidene malononitrile takes place through the Knoevenagel condensation followed by dehydration. The counter fluoride consumes the acidic proton of dimedone and this dimedone carbanion attack the electrophilic center of arylidene malononitrile to form a carbon- carbon bond,which on cyclization affords the desired product through tautomerization and proton transfer.
|
Download:
|
| Scheme. 3.A plausible mechanism of 2-amino-3-cyano-4H-pyran synthesis. | |
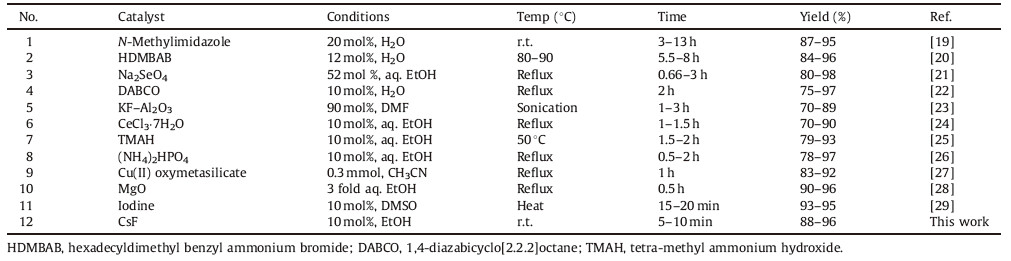
Finally,to manifest the efficiency and capability of the present protocol in the synthesis of different 2-amino-3-cyano-4H-pyran derivatives,it was compared with some of the previously reported and published procedures summarized results in Table 4. This comparison clearly illustrates the present method is indeed advantageous to several of the others in terms of high product yield,short reaction time,no additional energy required (i.e., heating,ultrasonication or microwaves),and easy isolation of final products by simple work-up.
| Table 4Comparative synthesis of 4H-pyran, the reported methods versus the present method. |
The syntheses of the 2-amino-3-cyano-4H-pyran derivatives were confirmed using FT-IR,1H-NMR spectroscopy. In the FT-IR spectra,the -NH2 signals were observed between 3450-3330 cm-1 and for -CN found in range of 2190-2200 cm-1. While,in the 1H NMR spectra,the -NH2 proton signals appeared as a broad singlet in the region of δ 6.79-7.10. The Ar-CH proton signals also appeared as a singlet in the region of δ 4.08-4.38 and the remaining proton signals were observed in the expected regions,confirming the formation of 2-amino-3-cyano-4H-pyran. A detailed description of the spectral data for compounds is provided in the Supporting information.
4. ConclusionIn conclusion,a series of biologically and pharmacologically active 2-amino-3-cyano-4H-pyran and spirooxindole derivatives have been synthesized by using CsF as catalyst via a one-pot,three component condensation of an aldehyde or isatin,malononitrile, and either 5,5-dimethyl-1,3-cyclohexanedione or 1,3-cyclohexanedione in excellent yields (82-96%) within a practical reaction time (5-10 min) at ambient temperature. Furthermore,the present method offers several advantages,such as mild conditions, quantitative yields of products in relatively short reaction times, elimination of refluxing conditions and simple work-up procedure, making this method an good alternative to the previous methodologies for the scale-up of these one-pot,three-component reactions. The exploration of CsF as catalyst for other multicomponent reactions leading to biologically active compounds is underway.
underway.AcknowledgmentsOne of the authors (Y.B.W.) acknowledges UGC,New Delhi for SAP fellowship under the scheme ‘Research Fellowship in Sciences for Meritorious Students’. The authors are also grateful to SAIF Panjab University for providing analytical facilities for characterization of compounds.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.06.014.
| [1] | B. Sharifzadeh, N.O. Mahmoodi, M. Mamaghani, et al., Facile regioselective synthesis of novel bioactive thiazolyl-pyrazoline derivatives via a three-component reaction and their antimicrobial activity, Bioorg. Med. Chem. Lett. 23(2013) 548-551. |
| [2] | R. Hosseinnia, M. Mamaghani, K. Tabatabaeian, F. Shirini, M. Rassa, An expeditious regioselective synthesis of novel bioactive indole-substituted chromene derivatives via one-pot three-component reaction, Bioorg. Med. Chem. Lett. 22(2012) 5956-5960. |
| [3] | N. Foroughifar, S. Ebrahimi, One-pot synthesis of 1,3-thiazolidin-4-one using bi(SCH2COOH)3 as catalyst, Chin. Chem. Lett. 24(2013) 389-391. |
| [4] | R. Baharfar, S.M. Baghbanian, Synthesis of novel uracil based 2,5-diaminofurans using multi-component reactions, Chin. Chem. Lett. 23(2012) 677-680. |
| [5] | L. Bonsignore, G. Loy, D. Secci, A. Calignano, Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivatives, Eur. J. Med. Chem. 28(1993) 517-520. |
| [6] | L.F. Tietze, Secologanin, a biogenetic key compound-synthesis and biogenesis of the iridoid and secoiridoid glycosides, Angew. Chem. Int. Ed. Engl. 22(1983) 828-841. |
| [7] | E.A.A. Hafez, M.H. Elnagdi, A.G.A. Elagamey, et al., Nitriles in heterocyclic synthesis:novel synthesis of benzo[c]coumarin and of benzo[c]pyrano[3,2-c]quinoline derivatives, Heterocycles 26(1987) 903-907. |
| [8] | (a) H. Hong, L.J. Huang, D.W. Teng, A spirocyclic oxindole analogue:synthesis and antitumor activities, Chin. Chem. Lett. 22(2011) 1009-1012;(b) D.-C. Wang, Y.-M. Xie, C. Fan, et al., Efficient and mild cyclization procedures for the synthesis of novel 2-amino-4H-pyran derivatives with potential antitumor activity, Chin. Chem. Lett. 25(2014) 1011-1013. |
| [9] | D. Kumar, V.B. Reddy, S. Sharad, et al., A facile one-pot green synthesis and antibacterial activity of 2-amino-4H-pyrans and 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromenes, Eur. J. Med. Chem. 44(2009) 3805-3809. |
| [10] | C.B. Sangani, D.C. Mungra, M.P. Patel, et al., Synthesis and in vitro antimicrobial screening of new pyrano[4,3-b]pyran derivatives of 1H-pyrazole, Chin. Chem. Lett. 23(2012) 57-60. |
| [11] | (a) W. Kemnitzer, S. Kasibhatla, S. Jiang, et al., Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based highthroughput screening assay. 2. Structure-activity relationships of the 7- and 5-, 6-, 8-positions, Bioorg. Med. Chem. Lett. 15(2005) 4745-4751;(b) S. Kasibhatla, H. Gourdeau, K. Meerovitch, et al., Discovery and mechanism of action of a novel series of apoptosis inducers with potential vascular targeting activity, Mol. Cancer Ther. 3(2004) 1365-1374. |
| [12] | D. Armesto, W.M. Horspool, N. Martin, et al., Synthesis of cyclobutenes by the novel photochemical ring contraction of 4-substituted 2-amino-3,5-dicyano-6-phenyl-4H-pyrans, J. Org. Chem. 54(1989) 3069-3072. |
| [13] | M. Chennapuram, N.R. Emmadi, C. Bingi, et al., Group-assisted purification (GAP) chemistry for dihydrofurans:water as a medium for catalyst free synthesis in a one pot four component reaction, Green Chem. 16(2014) 3237-3246. |
| [14] | A.R. Moosavi-Zare, M.A. Zolfigol, O. Khaledian, et al., Tandem Knoevenagel- Michael-cyclocondensation reactions of malononitrile, various aldehydes and dimedone using acetic acid functionalized ionic liquid, New J. Chem. 38(2014) 2342-2347. |
| [15] | M.G. Dekamin, M. Eslami, Highly efficient organocatalytic synthesis of diverse and densely functionalized 2-amino-3-cyano-4H-pyrans under mechanochemical ball milling, Green Chem. 16(2014) 4914-4921. |
| [16] | M.N. Elinson, A.S. Dorofeev, S.K. Feducovich, et al., The implication of electrocatalysis in MCR strategy:electrocatalytic multicomponent transformation of cyclic 1,3-diketones, aldehydes and malononitrile into substituted 5,6,7,8-tetrahydro-4H-chromenes, Eur. J. Org. Chem. 19(2006) 4335-4339. |
| [17] | V.M. Joshi, L.P.B. Rupali, Throat, et al., Novel one-pot synthesis of 4H-chromene derivatives using amino functionalized silica gel catalyst, Chin. Chem. Lett. 25(2014) 455-458. |
| [18] | (a) N. Azizi, S. Dezfooli, M.M. Hashemi, Synthesis of spirooxindole in deep eutectic solvent, J. Mol. Liq. 194(2014) 62-67;(b) Y. Li, H. Chen, C. Shi, et al., Efficient one-pot synthesis of spirooxindole derivatives catalyzed by L-proline in aqueous medium, J. Comb. Chem. 12(2010) 231-237;(c) M. Kidwai, A. Jahan, N.K. Mishra, et al., Gold(III) chloride (HAuCl4·3H2O) in PEG:a new and efficient catalytic system for the synthesis of functionalized spirochromenes, Appl. Catal. A 425(2012) 35-43;(d) B.M. Rao, G.N. Reddy, T.V. Reddy, et al., Carbon-SO3H:a novel and recyclable solid acid catalyst for the synthesis of spiro[4H-pyran-3,30-oxindoles], Tetrahedron Lett. 54(2013) 2466-2471. |
| [19] | X. Lian, Y. Huang, Y. Li, et al., A green synthesis of tetrahydrobenzo[b]pyran derivatives through three-component condensation using N-methylimidazole as organocatalyst, Monatsh. Chem. 139(2008) 129-131. |
| [20] | T.S. Jin, A.Q. Wang, F. Shi, et al., Hexadecyldimethyl benzyl ammonium bromide:an efficient catalyst for a clean one-pot synthesis of tetrahydrobenzopyran derivatives in water, ARKIVOC xiv (2006) 78-86. |
| [21] | R. Hekmatshoar, S. Majedi, K. Bakhtiari, Sodium selenate catalyzed simple and efficient synthesis of tetrahydro benzo[b]pyran derivatives, Catal. Commun. 9(2008) 307-310. |
| [22] | D. Tahmassebi, J. Bryson, S. Binz, 1,4-Diazabicyclo[2.2.2] octane as an efficient catalyst for a clean, one-pot synthesis of tetrahydrobenzo[b]pyran derivatives via multicomponent reactioninaqueousmedia, Synth.Commun.41(2011)2701-2711. |
| [23] | X. Wang, D. Shi, S. Tu, et al., Convenient synthesis of 5-oxo-5,6,7,8-tetrahydro-4Hbenzo-[b]-pyran derivatives catalyzed by KF-alumina, Synth. Commun. 33(2003) 119-126. |
| [24] | G. Sabitha, K. Arundhathi, K. Sudhakar, et al., Cerium(III) chloride-catalyzed onepot synthesis of tetrahydrobenzo[b]pyrans, Synth. Commun. 39(2009) 433-442. |
| [25] | S. Balalaie, M. Sheikh-Ahmadi, M. Bararjanian, Tetra-methyl ammonium hydroxide:an efficient and versatile catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran derivatives in aqueous media, Catal. Commun. 8(2007) 1724-1728. |
| [26] | S. Balalaie, M. Bararjanian, M. Sheikh-Ahmadi, et al., Diammonium hydrogen phosphate:an efficient and versatile catalyst for the one pot synthesis of tetrahydrobenzo[b]pyran derivatives in aqueous media, Synth. Commun. 37(2007) 1097-1108. |
| [27] | M.M. Heravi, Y.S. Beheshtiha, Z. Pirnia, et al., One-pot, three-component synthesis of 4H-pyrans using Cu(II) oxymetasilicate, Synth. Commun. 39(2009) 3663-3667. |
| [28] | M. Seifi, H. Sheibani, High surface area MgO as a highly effective heterogeneous base catalyst for three-component synthesis of tetrahydrobenzopyran and 3,4-dihydropyrano[c]chromene derivatives in aqueous media, Catal. Lett. 126(2008) 275-279. |
| [29] | R.S. Bhosale, C.V. Magar, K.S. Solanke, et al., Molecular iodine:an efficient catalyst for the synthesis of tetrahydrobenzo[b]pyrans, Synth. Commun. 37(2007) 4353-4357. |
| [30] | G.K. Friestad, B.P. Branchaud, W. Navarrini, M. Sansotera, Cesium fluoride, in:e-EROSEncyclopedia ofReagents forOrganicSynthesis, 2007, http://dx.doi.org/10.1002/047084289X.rc050.pub2. |
| [31] | K.P. Nandre, V.S. Patil, S.V. Bhosale, CsF mediated rapid condensation of 1,3-cyclohexadione with aromatic aldehydes:comparative study of conventional heating vs. ambient temperature, Chin. Chem. Lett. 22(2011) 777-780. |
| [32] | (a) Y.A. Tayade, D.R. Patil, Y.B. Wagh, et al., An efficient synthesis of 3-indolyl-3-hydroxy oxindoles and 3,3-di(indolyl)indolin-2-ones catalyzed by sulfonated β-CD as a supramolecular catalyst in water, Tetrahedron Lett. 56(2015) 666-673;(b) A.D. Jangale, P.K. Kumavat, Y.B. Wagh, et al., Green process development for the synthesis of aliphatic symmetrical N,N'-disubstituted thiourea derivatives in aqueous medium, Synth. Commun. 45(2015) 236-244;(c) D.R. Patil, Y.B. Wagh, P.G. Ingole, et al., β-Cyclodextrin-mediated highly efficient[2+3] cycloaddition reactions for the synthesis of 5-substituted 1Htetrazoles, New J. Chem. 37(2013) 3261-3270;(d) D.R. Patil, D.S. Dalal, Biomimetic approach for the synthesis of N,N'-diarylsubstituted formamidines catalyzed by β-cyclodextrin in water, Chin. Chem. Lett. 23(2012) 1125-1130. |
| [33] | K.M. Khan, I. Khan, S. Perveen, et al., A rapid and efficient CsF catalyzed tandem Knoevenagel-Michael reaction, J. Fluor. Chem. 158(2014) 1-5. |
 2015, Vol.26
2015, Vol.26