4A zeolite possesses an open three-dimensional porous structure,and the structure cell consists of SiO4/AlO4 tetrahedrons, called a sodalite cage. The sodalite cages are connected by single quadrangular rings to form supercages,which are the main channels of 4A zeolite. The extraframework cations (Na+) play a role of compensating charges for the negatively charged framework, and they interact with the framework through electrostatic attraction. The weak interaction determines the mobility of Na+ inside 4A zeolite. The transferring and hopping are two kinds of typical movement of Na+,and have been researched by molecular simulation methods [1, 2]. Meanwhile,the sensitive dielectric response of cation to environmental difference indicates that dielectric relaxation spectroscopy (DRS) can be used to explore the microscopic characteristics of zeolite under various environments [3, 4, 5]. Traditionally,the permittivity of porous materials,such as 4A zeolite,is obtained through electrical characterization of a sintered dense pellet. However,the structure and dielectric properties of the sintered products may differ from the original loose powders,especially for the materials with high sensitivity to mechanical and thermal treatment.
Recently,a so-called slurry method has been exploited to evaluate in situ the dielectric properties of powders with special structure [6, 7, 8]. In slurry method,the powders are dispersed in a special liquid dispersing medium,and the permittivity and conductivity of the bulk slurry is measured. By means of effective medium theory [7, 8, 9],the dielectric parameters of powders are calculated using the measured dielectric parameters of the slurries and medium. For slurry method,the selected medium should match with the dispersed powders. For example,propylene carbonate was considered to be an ideal medium for investigating the permittivity of barium titanate by slurry method. Compared with other organic liquids,the high permittivity (ε = 66.50,at 20 ℃) and the dipolar non-hydrogen-bond of propylene carbonate can compress effectively the error aroused by the huge permittivity (ε > 103) of barium titanate powders [10]. Different from solid powders,there are abundant pores inside 4A zeolite. The interaction between medium molecules and cations inside pore channels,which due to medium immerging into pore,has to be considered. For obtaining the original dielectric properties of porous powders,the diameter ratio of dispersing medium molecule and dispersed particle pore is another important factor.
In the present work,the dielectric responses of 4A zeolite bulk dispersion system to two dispersing mediums,water and cyclohexane,were investigated. Through fitting and calculating the dielectric properties of bulk dispersion systems with appropriate model function,the intrinsic structure of 4A zeolite are obtained and analyzed.
2. Experimental4A zeolite was commercially available from Sigma Chemical Co. The average particle size is around 4 μm and the density is about 2.07 g/cm3. Two kinds of bulk dispersion systems were prepared by dispersing ultrasonically 4A zeolite in deionized water (4A/W) and cyclohexane (4A/C),respectively. For 4A/W,the dispersing time exceeds 30 min in order the water can permeate inside zeolite pores,and then the soaking time exceeds 2 days in order that ionic exchange between two phases complete totally. Dielectric measurements were carried out on a Wayne Kerr 6500B Precision Impedance Analyzer with a component fixture 1011 (Wayne Kerr Electronics,UK) over a continuous frequency range of 20 Hz to 120 MHz. The amplitude of the applied alternating field was 500 mV. A dielectric measurement cell with parallel stainless steel electrodes was employed,and the volume of the dispersion system used in the experiment was 10 mL in order to submerge the electrodes. The temperature dependence of the dielectric properties was measured from 10 ℃ to 55 ℃ by circulating thermostated water,and the deviation of temperature was less than ±0.3 ℃.
3. Results and discussionFig. 1 shows the 3D representations of temperature dependent permittivity spectra of 4A/W and 4A/C respectively. The difference between relaxation behaviors of two bulk dispersion systems can be observed obviously. For comparing the medium effect on two systems quantificationally,the Cole-Cole equation with electrode polarization term (Eq. (1)) was applied to extract the dielectric parameters
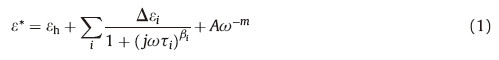
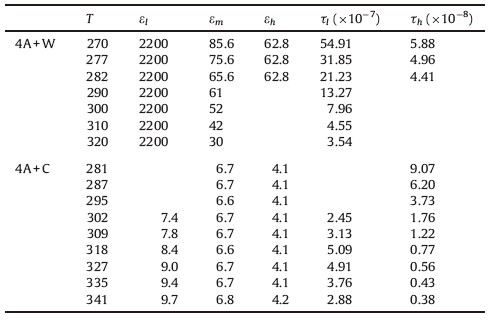
According to the fitting results listed in Table 1,the 4A/W system displays two dielectric relaxations at initial temperature 270 K,which locate at 104 Hz and 106 Hz respectively. The dielectric increment,Δε,was usually used to illuminate the intensity of the interfacial polarization of the heterogeneous dispersion systems [11]. Because of water immerging into pores, the interfacial properties of 4A/W deduced from dielectric increment in Table 1 cannot reflect the original character of 4A zeolite. The diameter of cyclohexane molecule is about 0.5 nm,and is larger than the pore diameter of 4A zeolite (0.4 nm). Although the 4A zeolite particles were dispersed in cyclohexane,the solvent molecules cannot enter the pores of zeolite particles,which still kept original. So Na+ in 4A/C is not affected by outside dispersing medium,and can be represented originally by the dielectric increment. According to Maxwell-Wagner equation as follows [12]:

| Table 1 Partial dielectric parameters of 4A/water and 4A/cyclohexane dispersion systems. |

|
Download:
|
| Fig. 1.Three-dimensional representations of temperature dependence of the permittivity spectra of (a) 4A/W and (b) 4A/C. | |

|
Download:
|
| Fig. 2.Temperature dependence of dielectric increment of 4A/C dispersion system. | |
With the temperature increasing as seen in Table 1,both of relaxations of 4A/W shifted to higher frequency. The temperature dependence of relaxation frequency can be explained by Arrhenius equation:


|
Download:
|
| Fig. 3.The temperature dependence of relaxation time of (a) 4A/W and (b) 4A/C. | |
Different from 4A/W system,HFR of 4A/C can be measured clearly from 281 K to 341 K. Comparing with the pure 4A zeolite, the interaction between Na+ and framework in 4A/C was not affected by the dispersing phase. So the activity of Na+ related to HFR is less much than that in 4A/W. According to Fig. 3b,the EA,HFR of 4A/C is about 43.6 kJ/mol,which is coincident with the data of dehydrated 4A zeolite in the prior study (49 kJ/mol). It indicates that the dispersing medium has no effect on the inner environment of zoelite. Among lower temperature under 300 K,however,there is no LFR found. With the temperature increasing above 300 K,the LFR displays nonlinear temperature dependence. The appearance of LFR can be considered as the result of activation of local Na+. Attained energy enough,part of Na+ ions can conquer the restriction from local framework,and started to transfer in supercages. As shown in Fig. 3b,however,the τ0 of LFR increases firstly and then decreases with temperature increasing from 302 K to 341 K. A transient state as shown in Fig. 4 was supposed to explain the abnormal changing of τLFR with increasing temperature. Under medium temperature,Na+ is inclined to escape from local sites,and tries to move along supercage. However,the increased activity of Na+ due to moderate temperature is not high enough to ensure Na+ to complete the full transferring movement in supercage (as Fig. 4b). So the Na+ was at semi-activated state, and the movement range is wider than hopping as Fig. 4a,and narrower than transferring. As seen in Fig. 3c,the transient movement induces a turbid response to the frequency.

|
Download:
|
| Fig. 4.Movements of Na+ in 4A zeolite at low frequency under different temperatures. | |
For 4A/C under transient state,the path for ions transferring is lengthened with increasing temperature. So Eq. (3),which can be used under constant transferring distance,is not suitable to calculate the t of LFR under transient state. Gross offered a model to describe the relaxation time related to the movement of ions as followed [15]:

| [1] | G.H. Zhai, P. Yang, S.M. Wu, Y.B. Lei, Y.S. Dou, A semiclassical molecular dynamics of the photochromic ring-opening reaction of spiropyran, Chin. Chem. Lett. 25(2014) 727-731. |
| [2] | J.Y. Wu, Q.L. Liu, Y. Xiong, A.M. Zhu, Y. Chen, Molecular simulation of water/alcohol mixtures' adsorption and diffusion in zeolite 4A membranes, J. Phys. Chem. B 113(2009) 4267-4274. |
| [3] | W. Zhou, K.S. Zhao, The study of dielectric properties of 4A zeolites dispersed in silicone oil, Colloid Surf. A 217(2008) 10-16. |
| [4] | G. Rodríguez-Fuentes, S. Devautour-Vinot, S. Diaby, F. Henn, Insights into cation exchange selectivity of a natural clinoptilolite by means of dielectric relaxation spectroscopy, Phys. Chem. Miner. 38(2011) 613-621. |
| [5] | A. Nicolas, S. Devautour-Vinot, G. Maurin, J.C. Giuntini, F. Henn, Cation dynamics upon adsorption of methanol in Na-Y faujasite type zeolites:A dielectric relaxation spectroscopy investigation, J. Phys. Chem. C 111(2007) 4722-4726. |
| [6] | V. Petrovsky, T. Petrovsky, S. Kamlapurkar, F. Dogan, Characterization of dielectric particles by impedance spectroscopy (Part I), J. Am. Ceram. Soc. 91(2008) 1814-1816. |
| [7] | V. Petrovsky, T. Petrovsky, S. Kamlapurkar, F. Dogan, Physical modeling and electrodynamic characterization of dielectric slurries by impedance spectroscopy (Part II), J. Am. Ceram. Soc. 91(2008) 1817-1819. |
| [8] | W. Zhou, B.B. Hinojosa, J.C. Nino, Applicability of the Bruggeman equation for analyzing dielectric slurries containing ceramic powders with high permittivity, J. Am. Ceram. Soc. 95(2012) 457-460. |
| [9] | W. Zhou, Y.M. Nie, S.J. Li, H.Y. Liang, The importance of the solids loading on confirming the dielectric nanosize dependence of BaTiO3 powders by slurry method, Sci. World J. 2013(2013) 1-3. |
| [10] | S. Wada, H. Yasuno, T. Hoshina, et al., Preparation of nm-sized barium titanate fine particles and their powder dielectric properties, Jpn. J. Appl. Phys. 42(2003) 6188-6195. |
| [11] | A.V. Delgado, F.J. Arroyo, F. González-Caballero, V.N. Shilov, Y.B. Borkovskaya, The effect of the concentration of dispersed particles on the mechanisms of lowfrequency dielectric dispersion (LFDD) in colloidal suspensions, Colloid Surf. A 140(1998) 139-149. |
| [12] | T. Hanai, K. Sekine, Theory of dielectric relaxations due to the interfacial polarization for two-component suspensions of spheres, Colloid Polym. Sci. 264(1986) 888-895. |
| [13] | B. Legras, I. Polaert, L. Estel, M. Thomsa, Mechanisms responsible for dielectric properties of various faujasites and linde type A zeolites in the microwave frequency range, J. Phys. Chem. C 115(2011) 3090-3098. |
| [14] | T. Ohgushi, K. Ishimaru, Dielectric properties of dehydrated NaA zeolite, analyses and calculation of dielectric spectra, Phys. Chem. Chem. Phys. 3(2001) 3229-3234. |
| [15] | C. Grosse, Permittivity of a suspension of charged spherical particles in electrolyte solution. 2. Influence of the surface conductivity and asymmetry of the electrolyte on the low- and high-frequency relaxations, J. Phys. Chem. 92(1988) 3905-3910. |
 2015, Vol.26
2015, Vol.26