b Center of Physical and Chemistry Test, Shenyang University of Chemical Technology, Shenyang 110142, China
Mercury pollution is a global problem,and a major source of human exposure stems from contaminated waters [1, 2]. The terrific toxicity of mercury results from its high affinity for thiol groups in proteins and enzymes,leading to the dysfunction of cells and consequently causing health problems [3]. Inorganic mercury,including Hg(0) and Hg(II),is released into the environment through a wide variety of ways. Industrial sources of mercury include coal and gold mining,solid waste incineration,wood pulping,fossil fuel combustion,and chemical manufacturing. As the results of numerous applications in industry and agriculture,mercury has badly contaminated rivers and soil and accumulated in agricultural products and aquatic products,which causes serious environmental and health problems directly or indirectly [4]. The U.S. Environmental Protection Agency (EPA) standard for the maximum allowable level of inorganic mercury in drinking water is 2 ppb [5]. Moreover,a significant problem stemming from the ecological oxidation chemistry is that bacteria living in the sediments of aqueous environments transform inorganic Hg2+ into methylmercury,a potent neurotoxin that concentrates through the food chain in the tissues of fish and marine mammals [6]. Subsequent ingestion of methylmercury by humans from seafood and other dietary and environmental sources is connected to serious sensory,motor,and cognitive disorders. Therefore,in the interests of safety the detection of Hg2+ is still of great importance.
Among the available techniques to detect and quantify Hg2+,colorimetric chemosensors,so-called ‘‘naked eye chemosensors’’,have been developed and are attracting increasing interest [7, 8]. This sensing approach has clear potential application in the development of commercial Hg2+ indicators,such as paper test strips that can be evaluated visually without illumination [7, 8]. Water solubility is arguably a less important criterion for colorimetric chemosensors than for fluorescent ones,which are better suited for in vivo work [9, 10]. As long as the colorimetric indicator responds to Hg2+ in the presence of water and can be absorbed onto a paper strip or into a membrane or film,it has potential. Therefore,we herein communicate our studies on colorimetric Hg2+ detection,based on the new water-soluble PODIPY dye.
Our recent research interest lies in the novel BODIPY/aza- BODIPY family of fluorescent dyes and their application [11, 12, 13],and very recently we developed a type of PODIPY dye [14]. The new PODIPY 1 (Fig. 1) can be successfully synthesized by the reaction of dipyrromethene and azadipyrromethene with POCl3 in the presence of Et3N. The new PODIPY had photophysical properties similar to related PODIPYs/aza-PODIPYs [15, 16, 17, 18, 19, 20, 21],with a large Stokes shift and moderate fluorescence quantum yield. The dye 1 is more polar than BODIPYs,increasing its water solubility. Moreover,the interaction between the p-orbital of the phosphorus atom and the lone pair electrons of the nitrogen atom,and many resonators of the limit structure of charge separation in dye 1,may also enhance the stability in water. Based on the photophysical properties of 1,we made further efforts for the modifications in PODIPY as potential chemosensors and probes. Because of the low fluorescence quantum yield (Φf = 0.17) of PODIPY 1 [14] and the additional fluorescence quenching by detecting Hg2+,the investigation of emission spectra becomes meaningless and was abandoned. Delightedly,we found that PODIPY 1 can be used as a chemosensor for the colorimetric Hg2+ detection.

|
Download:
|
| Fig. 1.Structure of PODIPY dye 1. | |
1H NMR spectra were recorded on a Bruker AVANCE III 500 MHz spectrometer. 1H NMR chemical shifts (δ) are given in ppm downfield from Me4Si,determined by chloroform (δ = 7.26 ppm). 13C NMR spectra were recorded on a Bruker AVANCE III 125 MHz spectrometer. 13C NMR chemical shifts (δ) are reported in ppm with the internal CDCl3 at δ 77.0 ppm as standard. ESI was measured by LCQ Deca XP. Tetrahydrofuran (THF) was freshly distilled from Na/benzophenone,n-hexane was distilled over Na,and other solvents were distilled over CaH2. Merck silica gel 60 was used for the column chromatography. Fluorescence spectra were recorded on an F-4600 spectrophotometer. UV/Vis spectra were recorded on a UV-2550 spectrophotometer at room temperature. The pH measurement was performed with a PHS-3E pH meter. The refractive index of the medium was measured by 2W Abbe’s refractometer at 20 ℃.
2.2. Synthesis of PODIPY 12.4-Dimethylpyrrole (0.51 mL,4.9 mmol) and paranitrobenzaldehyde (300 mg,1.9 mmol) were dissolved in 20 mL of absolute CH2Cl2 under a N2 atmosphere. One drop of trifluoroacetic acid (TFA) was added and the solution was stirred at room temperature overnight. When TLC monitoring (silica; CH2Cl2) showed complete consumption of the benzaldehyde,a solution of 2,3-dichloro-5,6- dicyano-1,4-benzoquinone (DDQ) (900 mg) in CH2Cl2 (10 ml) was added,and stirring was continued for 1 h. The reaction mixture was washed with water,dried over MgSO4,filtered,and evaporated. The crude compound was purified by column chromatography on aluminum (CH2Cl2/n-hexane,1:1). This solid and triethylamine (2 mL) were dissolved in 50 mL of CH2Cl2 under air and the solution was stirred at room temperature for 10 min. POCl3 (4 mL) was added,and stirring was continued for 1 h. The reaction was slowly quenched with crushed ice,extracted with CH2Cl2,and purified by recrystallization from CH2Cl2/n-hexane to afford 1 (171.2 mg,23.5%) as red solids. 1H NMR (500 MHz,CDCl3): δ 8.37 (d,2H,J = 8.0 Hz),7.56 (d,2H,J = 8.0 Hz),6.24 (s,2H),2.56 (s,6H),1.39 (s,6H). 13C NMR (125 MHz,DMSO-d6): δ 156.5,153.2,142.6,133.9,131.5,129.5,123.8,120.8,99.3,13.8,13.7. 31P NMR (202 MHz,CDCl3): δ -50.011. FTMS-MALDI (m/z): calcd. for C19H19N3O4P: 384.1113 [M+H]+,found: 384.1085; calcd. for C19H19N3O2: 322.1511 [M-PO2+H]+; found: 322.1525.
2.3. Preparation of metal ion titration solutionsStock solutions (4 × 10-4 mol/L) of the salts of HgCl2,Al(NO3)3,AgNO3,CoCl2,MnCl2,PbCl2,CuCl2,MgCl2,NiCl2,FeCl3,ZnCl2,CaCl2,PdCl2,CrCl3,KCl,and NaCl in H2O were prepared. PODIPY 1 (1 × 10-4 mol/L) was also prepared in CH3CN. Test solutions were prepared by placing 40 μL of the sensor stock solution into a test tube,then adding an appropriate aliquot of each metal stock (0 - 1.0 mL) and diluting the solution to 4 mL with CH3CN/H2O (1/9,v/v).
2.4. MO calculationFrontier molecular orbitals have been performed at the Becke3LYP (B3LYP) level of the density functional theory. The SDD basis set are used to describe Hg and P and 6-31G(d) basis set was used for all the other atoms (see Supporting information).
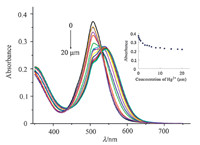
3. Results and discussionThe sensitivity of phosphorus-containing PODIPY 1 (λabs = 507 nm) was first studied by the UV-visible absorption response toward various concentrations of Hg2+ in CH3CN/H2O at pH 7.2 (Fig. 2) [22]. As can be observed,a distinct response of 1 μmol/L PODIPY 1 to Hg2+ in the concentration range of 0- 20 μmol/L was discovered. When 10 equiv. of Hg2+ (10 μmol/L) was added,the reduction of the absorption intensity (λabs = 550 nm) was minimal,and a further increase in of Hg2+ concentration did not provide further reduction (Fig. 2). Based on a linear range for Hg2+ covering from 0.1 μmol/L to 3 μmol/L (inner panel of Fig. 2),the detection limit (3σ/k) was found to be 0.5 μmol/L [23].
|
Download:
|
| Fig. 2.Absorption change of 1 μmol/L PODIPY 1 after the addition of increasing amounts of Hg2+ (0, 0.1, 0.25, 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 15, 18 and 20 μmol/L) in CH3CN/H2O (1:9, v/v) at room temperature. The inner panel displays the absorption intensity (λabs = 507 nm) of 1 μmol/L PODIPY 1 toward Hg2+ at 0, 0.1, 0.25, 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 15,18 and 20 μmol/L. | |
In fact,the presence of Hg2+,can be detected without the use of any instrumentation as illustrated in Fig. 3,with the color change from pink to violet red. The selectivity toward mercury has been demonstrated even in the presence of similar amounts of other metal ions,such as Na+,K+,Mg2+,Al3+,Cu2+,Fe3+,Zn2+,Ba2+,Ag+,Mn2+,Ni2+,Co2+,Cr3+,Pb2+ and no metal (Fig. 3).

|
Download:
|
| Fig. 3. Digital picture of PODIPY 1 (1 μmol/L) in CH3CN/H2O (1:9, v/v). From left to right: Na+, K+, Mg2+, Al3+, Cu2+, Fe3+, Zn2+, Ba2+; no-metal, Hg2+, Ag+, Mn2+, Ni2+, Co2+, Cr3+, Pb2+, equivalent amounts of all the cations and Hg2+. The cation total concentration was 10 μmol/L. | |
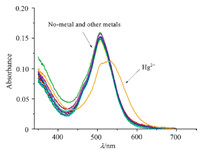
Furthermore,the selectivity of the chemosensor 1 toward other metal ions was investigated with UV-visible spectroscopy. As shown in Fig. 4,PODIPY 1 was highly selective to Hg2+ with a remarkable absorption change. In contrast,addition of other relevant metal ions,including Na+,K+,Mg2+,Al3+,Cu2+,Fe3+,Zn2+,Ba2+,Hg2+,Ag+,Mn2+,Ni2+,Co2+,Cr3+,and Pb2+,caused almost no absorption change.
|
Download:
|
| Fig. 4. Absorption spectra of PODIPY 1 (0.5 μmol/L) before and after the addition of metal salts (10 μmol/L) of Na+, K+, Mg2+, Al3+, Cu2+, Fe3+, Zn2+, Ba2+, Hg2+, Ag+, Mn2+, Ni2+, Co2+, Cr3+, Pb2+ in CH3CN/H2O (1:9, v/v). | |
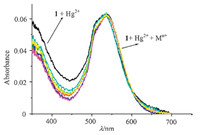
Some ions,for example,Zn2+,Ag+,Fe3+,Ni2+,Cr3+,or Pb2+,often interfere with the selectivity of Hg2+ [24, 25]. Therefore,to investigate the selectivity for Hg2+ in a complex background of potentially competing species,the absorption change of PODIPY 1 with Hg2+ was examined in the presence of other metal ions. From Fig. 5,we observed that there was no interference in the detection of Hg2+ in the presence of Zn2+,Ag+,Fe3+,Ni2+,Cr3+,or Pb2+,and the absorption spectra were almost identical to those obtained in the presence of Hg2+ alone.
|
Download:
|
| Fig. 5. The relative absorption of PODIPY 1 and its complexation with Hg2+ in the presence of various metal ions. PODIPY 1 + Hg2+; PODIPY 1 + Hg2++Mn+, where Mn+ = Zn2+, Ag+, Fe3+, Ni2+, Cr3+, and Pb2+ ions. Conditions: 0.3 μmol/L of PODIPY 1, 5 μmol/L of Hg2+ in the presence of 5 μmol/L of other metal ions. | |
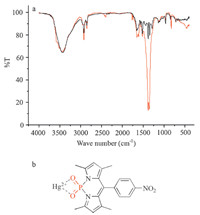
The recognition mechanism of the chemosensor 1 with Hg2+ was investigated by FT-IR spectra (Fig. 6a). 1 equiv. of Hg2+ with PODIPY 1 (red curve) intensified and widened the peak at the characteristic stretching frequency (1346.2/cm) [26] of the P=O bond of POPDIPY 1 alone (black curve). This indicated a strong polarization of the P=O bond upon efficient binding to the Hg2+ ion (Fig. 6b).
|
Download:
|
| Fig. 6. (a) IR spectra of compound PODIPY 1 (black curve) and PODIPY 1–Hg2+ complex (red curve) in KBr disks. (b) Structure of 1–Hg2+. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) | |
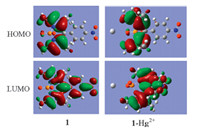
To gain insight into the response of chemosensor 1 to Hg2+,chemosensor 1 and the corresponding metal complex species 1- Hg2+ were examined by density function theory (DFT) at the Becke3LYP (B3LYP) level of the Gaussian 03 program. The SDD basis set are used to describe Hg and P,and 6-31G(d) basis set was used for all the other atoms. As shown in Fig. 7,for chemosensor 1,the HOMO are distributed at the PODIPY core,and the LUMO are localized on the whole PODIPY structure including the pnitrophenyl group in the neutral compounds. In addition,the planar BODIPY fragment is almost coplanar to the adjacent pnitrophenyl part with a small dihedral angle (4°). While for the metal complex species 1-Hg2+,the HOMO is distributed in the left BODIPY core,and the LUMO is mostly located at the right BODIPY unit. Furthermore,the energy gap between the HOMO and LUMO of the metal complex species 1-Hg2+ (2.04 eV) is smaller than that of chemosensor 1 (2.18 eV) (Fig. 7),which is in good agreement with the red shift in the absorption observed upon treatment of chemosensor 1 with Hg2+.
|
Download:
|
| Fig. 7. Frontier molecular orbitals of 1 and 1–Hg2+ at the Becke3LYP (B3LYP) level of the density functional theory with Gaussian 03. The SDD basis set is used to describe Hg and P, and 6-31G(d) basis set was used for all the other atoms. HOMO/LUMO (eV) = -5.96/-3.78 for 1; HOMO/LUMO (eV) = -5.84/-3.80 for 1–Hg2+. | |
Motivated by the obvious color change of the system in metal ions,test strips were prepared by immersing filter papers in the CH3CN/H2Osolution of1 (1.0 × 10-5 mol/L) and then drying themin air. When each drop of 5.0× 10-5 mol/L different metal ions was dripped into the 1-based test strips,a clear color change from pink to brown was observed in the presence of Hg2+,comparing to noncolor change for the other metal ions,such as Al3+,Cr3+ Ba2+,Pb2+ Ni2+ Mn2+,Cu2+,Ag+ and free (Fig. 8). Therefore,the 1-based test strips can conveniently detect Hg2+ in solutions without any additional equipment. The 1-based test strips were easily fabricated and low-cost,useful in practical and efficient Hg2+ test kits.

|
Download:
|
| Fig. 8. Photographs of test strips 1 to one drop of different metal ions. Test strips were prepared by immersing filter papers in the CH3CN/H2O (1/1, v/v) solution of 1 (1.0 × 10-5 mol/L) and then drying them in air. | |
PODIPY 1 can detect Hg2+ by the color change from pink to violet red without the use of any instrumentation. PODIPY 1 was selective to Hg2+ with remarkable absorption change,and the addition of other relevant metal ions caused almost no absorption change. The phosphorus-containing PODIPY 1 was sensitive to various concentrations of Hg2+. From the changes in Hg2+- dependent absorption intensity,the detection limit was found to be 0.5 mmol/L. Using PODIPY 1 there was no interference in the detection of Hg2+ in the presence of other ions. FT-IR indicated a strong polarization of the P55O bond in PODIPY 1 upon efficient binding to Hg2+. The energy gap between the HOMO and LUMO of the metal complex 1-Hg2+ (2.04 eV) is smaller than that of chemosensor 1 (2.18 eV),which is in good agreement with the red shift in the absorption observed upon treatment of sensor 1 with Hg2+. The 1-based test strips were easily fabricated and low-cost,useful in practical and efficient Hg2+ test kits. Further efforts for development of probes based on PODIPY/aza-PODIPY in biotechnology are ongoing in our lab.
AcknowledgmentsThis work was supported by the Public Research Foundation of Liaoning Province for the Cause of Science (No. 2014003009),Science and Technology Key Project of Liaoning Province (No. 2013304007),the Foundation of the Education Department of Henan Province for Science and Technology Research Projects (No. 13A150046),the Scientific Research Foundation for the Returned Overseas Chinese Scholars,State Education Ministry,and the startup funds from Shenyang University of Chemical Technology.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.07.002.
| [1] | E.M. Nolan, S.J. Lippard, Tools and tactics for the optical detection of mercuric ion, Chem. Rev. 108 (2008) 3443–3480. |
| [2] | R. Joseph, C.P. Rao, Ion and molecular recognition by lower rim 1, 3-di-conjugates of calix[4]arene as receptors, Chem. Rev. 111 (2011) 4658–4702. |
| [3] | H.H. Harris, I.J. Pickering, G.N. George, The chemical form of mercury in fish, Science 301 (2003) 1203–11203. |
| [4] | D.W. Domaille, E.L. Que, C.J. Chang, Synthetic fluorescent sensors for studying the cell biology of metals, Nat. Chem. Biol. 4 (2008) 168–175. |
| [5] | Mercury Update: Impact on Fish Advisories; EPA Fact Sheet EPA-823-F-01-001, Environmental Protection Agency, Office of Water, Washington, DC, 2001. |
| [6] | Z. Han, B. Zhu, T. Wu, et al., A fluorescent probe for Hg2+ sensing in solutions and living cells with a wide working pH range, Chin. Chem. Lett. 25 (2014) 73–76. |
| [7] | Z.Q. Yan, S.Y. Guang, H.Y. Xu, X.Y. Liu, An effective real-time colorimeteric sensor for sensitive and selective detection of cysteine under physiological conditions, Analyst 136 (2011) 1916–1921. |
| [8] | J.S. Lee, M.S. Han, C.A. Mirkin, Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles, Angew. Chem. Int. Ed. 46 (2007) 4093–4096. |
| [9] | J.R. Lakowicz, Principles of Fluorescence Spectroscopy, 3rd ed., Springer, Heidelberg, 2006. |
| [10] | F. Bergstroem, I. Mikhalyov, P. Haeggloef, et al., Dimers of dipyrrometheneboron difluoride (BODIPY) with light spectroscopic applications in chemistry and biology, J. Am. Chem. Soc. 124 (2002) 196–204. |
| [11] | X.D. Jiang, J. Zhang, T. Furuyama, W. Zhao, Development of mono- and di-AcO substituted BODIPYs on the boron center, Org. Lett. 14 (2012) 248–251. |
| [12] | X.D. Jiang, D. Xi, J. Zhao, et al., A styryl-containing aza-BODIPY as near-infrared dye, RSC Adv. 4 (2014) 60970–60973. |
| [13] | P. Shi, X.D. Jiang, R. Gao, et al., Synthesis and application of Vis/NIRdialkylaminophenylbuta- 1,3-dienyl borondipyrromethene dyes, Chin. Chem. Lett. 26 (2015) 834–838. |
| [14] | X.D. Jiang, J. Zhao, D. Xi, et al., A new water-soluble phosphorus-dipyrromethene and phosphorus-azadipyrromethene dye: PODIPY/aza-PODIPY, Chem. Eur. J. 21 (2015) 6079–6082. |
| [15] | A. Loudet, K. Burgess, BODIPY dyes and their derivatives: syntheses and spectroscopic properties, Chem. Rev. 107 (2007) 4891–4932. |
| [16] | L. Yuan, W. Lin, K. Zheng, L. He, W. Huang, Far-red to near infrared analyteresponsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging, Chem. Soc. Rev. 42 (2013) 622–661. |
| [17] | Y. Yang, Q. Zhao, W. Feng, F. Li, Luminescent chemodosimeters for bioimaging, Chem. Rev. 113 (2013) 192–270. |
| [18] | S.-B. Yi, H.-F. Gao, Q. Li, Y.-F. Ye, et al., Synthesis and self-assembly behavior of 2,5- diphenylethynyl thiophene based bolaamphiphiles, Chin. Chem. Lett. 26 (2015) 872–876. |
| [19] | P.-Z. Chen, H.-R. Zheng, L.-Y. Niu, et al., A BODIPY analogue from the tautomerization of sodium 3-oxide BODIPY, Chin. Chem. Lett. 6 (2015) 631–635. |
| [20] | M. Sun, H. Nie, J. Yao, Y. Zhong, Bis-triarylamine with a cyclometalated diosmium bridge: a multi-stage redox-active system, Chin. Chem. Lett. 6 (2015) 649–652. |
| [21] | Y. Wu, C. Cheng, L. Jiao, et al., β-Thiophene-fused BF2-azadipyrromethenes as near-infrared dyes, Org. Lett. 16 (2014) 748–751. |
| [22] | K. Huang, H. Yang, Z. Zhou, et al., A highly selective phosphorescent chemodosimeter for cysteine and homocysteine based on platinum(II) complexes, Inorg. Chim. Acta 362 (2009) 2577–2580. |
| [23] | M. Chen, X. Lv, Y. Liu, et al., An 2-(2'-aminophenyl)benzoxazole-based off–on fluorescent chemosensor for Zn2+ in aqueous solution, Org. Biomol. Chem. 9 (2011) 2345–2349. |
| [24] | X. Chen, T.H. Pradhan, F. Wang, J.S. Kim, J.Y. Yoon, Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives, Chem. Rev. 112 (2012) 1910–1956. |
| [25] | M.N. Elizabeth, J.L. Stephen, Turn-on fluorescent sensor for the selective detection of mercuric ion in aqueous media, J. Am. Chem. Soc. 125 (2003) 14270–14271. |
| [26] | H.G. Brittain, Physical Characterization of Pharmaceutical Solids, Marcel Dekker, New York, 1995. |
 2015, Vol.26
2015, Vol.26 


