b Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology(Ministry of Education), Department of Chemistry, Tsinghua University, Beijing 100084, China
Cu-catalyzed arylation reactions are very practical and efficient to transfer aromatic halides into more valuable aromatic compounds[1].Although a large variety of hetero-atomed (N,O and S) nucleophiles are generally applied as the partner of aromatic halides in these reaction[2, 3, 4],C-nucleophiles are limited to highly activated carbonyl compounds such as keto-esters,malonic acid derivatives,or diketones[5].Very recently,the first example of Cu-catalyzed α-arylation of benzyl phenyl ketones with aromatic iodides was also realized[6].The Cu-catalyzed arylation of simple ketones (such as methyl ketones) is very attractive but remains a big challenge[7].On the other hand,only aromatic iodides (other than bromides and chlorides) gave satisfactory results in most Cu-catalyzed arylation reactions.
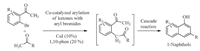
As one part of our ongoing project on synthesizing cyclic compounds with copper catalysts[8],recently,we reported a specific example that Cu-catalyzed arylation reaction of simple methyl ketones with o-iodoacetophenones was realized under very mild conditions and a tandem cyclization followed to give useful 1-naphthol derivatives[9].After great effort,the reaction was found to be applicable to o-bromoacetophenones,which was initiated by a rare Cu-catalyzed arylation reaction of methyl ketones with aromatic bromides (Scheme 1)!Since substituted o-bromoacetophenones are more economical,readily available but less reactive than corresponding o-iodoacetophenones,it is worth reporting that the Cu-catalyzed arylation reactions of simple methyl ketones with o-bromoacetophenones and subsquential cyclization reactions to 1-naphthols[10].
|
Download:
|
| Scheme. 1.Facile synthesis of 1-naphthols through a copper-catalyzed arylation of methyl ketones with o-bromoacetophenones. | |
All the reactions were carried out in a pre-dried screwcapped tube with a Teflon-lined septum under N2 atmosphere.Bromo acetophenone derivatives except bromoacetophenone 1a were prepared according to the literatures.All of the solvents were freshly distilled.Column chromatography was performed on silica gel (particle size 10-40 μm,Ocean Chemical Factory of Qingdao,China).1H NMR and 13C NMR spectra were recorded on a JEOL AL-300 MHz or AL-400 MHz spectrometer at ambient temperature with CDCl3 as the solvent.Chemical shifts (δ) were given in ppm,referenced to the residual proton resonance of CDCl3(7.26),to the carbon resonance of CDCl3(77.16).Coupling constants (J) were given in Hertz (Hz).The term m,dq,q,t,d,s referred to multiplet,doublet quartet,quartet,triplet,doublet,singlet.Mass spectra were obtained using Bruker Esquire ion trap mass spectrometer in positive mode.The reaction progress was monitored by GC-MS if applicable,using n-dodecane as internal standard.
2.1.General procedure for the preparation of desired compound 3a-lA sealed tube was charged with the mixture of CuI (0.1 mmol,19.0 mg),1,10-phenanthroline (0.2 mmol,36.0 mg),t-BuONa (6 mmol,0.576 g) and bromo acetophenone derivatives 1(1.0 mmol).The tube was evacuated and recharged with N2 for 3 times.Before toluene (2.0 mL) was added,the tube was sealed and the mixture was allowed to stir at 120 ℃ for 4 h.After completion,the mixture was cooled to room temperature,then 2 mol/L HCl aq.(1-2 mL) was added and the mixture was extracted with Et2O (5mL × 3),dried over anhydrous Na2SO4.Evaporation of the solvent followed by purification on silica gel (petroleum ether/diethyl ether:25/1 to 10/1) provided the corresponding product 3a-l as a solid or yellow oil.
2.2.General procedure for the preparation of desired compound 3m-qA sealed tube was charged with the mixture of CuI (0.1 mmol,19.0 mg),1,10-phenanthroline (0.2 mmol,36.0 mg),t-BuONa (6 mmol,0.576 g) and o-bromoacetophenone 1a (1.0 mmol).Thetube was evacuated and recharged with N2 for 3 times.After toluene (2.0 mL) was added,ketone 1m-q (3 equiv.,3 mmol) was added to the tube slowly.Then the tube was sealed and the mixture was allowed to stir at 120 ℃ for 4 hours.After completion,the mixture was cooled to room temperature,then 2 mol/L HCl aq.(1-2 mL) was added and the mixture was extracted with Et2O (5 mL × 3),dried over anhydrous Na2SO4.Evaporation of the solvent followed by purification on silica gel (petroleum ether/diethyl ether:25/1 to 10/1) provided the corresponding product 3m-q as a solid.
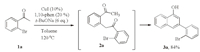
3.Results and discussionThe beginning study stemmed from the synthesis 1-naphthols via dimerization of o-bromoacetophenones 1a.We attempted to carry out the reaction under the same reaction as used in the case of o-iodoacetophenones but failed to get any product 2a[9].Considering the lower reactivity of o-bromoacetophenones,the reaction temperature was raised to 80 ℃ with THF as the solvent.Excitingly,the expected product 2a was formed in 20% yield.It is worth noting that t-BuONa was found as the best base for this reaction and the amount of t-BuONa was crucial:2a was formed in 58% yield with 5 equiv.of t-BuONa,and in 70% yield with 6 equiv.of t-BuONa (See Table S1 in Supporting information).When the reaction was carried out in toluene at 120 ℃,3a was isolated in 84% yield after normal work-up (Scheme 2)(For the details of optimization,see Supporting information).
|
Download:
|
| Scheme. 2.The best condition for the formation of 3a. | |
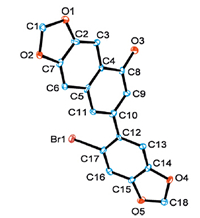
Inspired by the above result,a series of substituted obromoacetophenones 1 were homo-dimerized to give functionalized 1-naphthols 3,and the results were listed in Fig.1.Generally,o-bromoacetophenones 1 with a range of substitutents such as methyl,methoxide,fluoro,and bromo groups (1b-1j) could perform the reactions well to give substituted phenyl-1-naphthols (3b-3j).Excitedly,when polycyclic substrates (1k-1l) were used,ring-fused 1-naphthols were obtained (3k-3l).The structure of 3l was unequivocally confirmed with X-ray diffraction (Fig.2).
|
Download:
|
| Fig. 1.1-Naphthols 3 formed by the dimerization of 1. | |
|
Download:
|
| Fig. 2.Crystal structure of 7-(6-bromobenzo[d][1,3]dioxol-5-yl)naphtho[2,3-d][1,3]dioxol-5-ol 3l (CCDC number: 935169). Hydrogen atoms are omitted for clarity. | |
Encouraged by the successful synthesis of 1-naphthols via the homo-dimerization of o-bromoacetophenones 1,we attempted to realize the cross-cyclization of o-bromoacetophenone 1a with other simple ketones.Apparently,this reaction is more challenging since the homo-dimerization of o-bromoacetophenones should be avoided.To our delight,as shown in Table 1,it was found whensimple ketones 1m-1q were used much excess to o-bromoacetophenone 1a,only cross-cyclization products were isolated.Interestingly,when asymmetric ketone,2-butanone 1n was employed,only 2-ethyl-1-naphthol 3n was isolated,which indicated CH3 group was more reactive than CH2 group in this reaction.Propiophenone 1q was also fit for this reaction to produce 3,4-disubstituted 1-naphthol 3q,albeit in a lower yield.
| Table 1 1-Naphthols 3m-3q formed by the reaction of 1a with methyl ketones. |
We also attempted the reaction of o-iodine acetophenone with 1o-1q according to the condition in Table 1.Unfortunately,under this condition,the reaction gave messy products due to the strong reactivity of o-iodine acetophenone.
It is worth noting that those synthesized 1-naphthols 3a-3l with one bromo-atom were able to be further modified.For example,compound 3a was reduced to give 3o by the treatment with HCOONH4 solution in the presence of Pd/C (10%)(Scheme 3)[11].Under a standard Suzuki-coupling condition with phenylboronic acid,compound 3a was successfully converted to 3r in 75% yield (Scheme 4)[12].Moreover,the modification of compound 3a was sometimes able to be done in one pot during its formation from 1a (Scheme 5).When 1a was reacted with trifluoroethanol in the standard condition[13],product 3s was isolated in 78% yield.

|
Download:
|
| Scheme. 3.The reductive of 1-naphthol 3a in the presence of Pd/C. | |

|
Download:
|
| Scheme. 4.Suzuki-coupling of 1-naphthol 3a and phenylboronic acid. | |
|
Download:
|
| Scheme. 5.Synthesis of compound 3-(2-(2,2,2-trifluoroethoxy)phenyl)naphthalen-1-ol 3s in one pot from 1-naphthol 3a. | |
In conclusion,we have developed Cu-catalyzed cyclization reaction for the synthesis of 1-naphthols by the dimerization of obromoacetophenones 1 or cross-cyclization of o-bromoacetophenones 1 with simple ketones 1m-1q.The process was initiated by a rare Cu-catalyzed arylation of simple methyl ketones with aromatic bromides.The cascade strategy with simple substrates and catalyst marks a significant departure from known approaches.Therefore,we believe this method will be very useful in organic chemistry and medicinal chemistry.
AcknowledgmentThis work was supported by National Natural Science Foundation of China (Nos.21102080,21372138) and Tsinghua University Initiative Scientific Research Program (No.2011Z02150).
Appendix A.Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.07.022.
| [1] | (a) For reviews on the catalytic Ullmann reaction, see:F. Monniers, M. Taillefer, Catalytic C-C, C-N, and C-O Ulmann-type coupling reactions, Angew. Chem. Int. Ed. 48(2009) 6954-6971;(b) G. Evano, N. Blanchard, M. Toumi, Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis, Chem. Rev. 108(2008) 3054-3131;(c) A.W. Thomas, S.V. Ley, Modern synthetic methods for copper-mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S bond formation, Angew. Chem. Int. Ed. 42(2003) 5400-5449. |
| [2] | (a) Cu-catalyzed arylation reactions with N-nucleophiles, see:F. Ullmann, Ueber eine neue bildungsweise von diphenylaminderivaten, Chem. Ber. 36(1903) 2382-2384;(b) A. Kiyomori, J.F. Marcoux, S.L. Buchwald, An efficient copper-catalyzed coupling of aryl halides with imidazoles, Tetrahedron Lett. 40(1999) 2657-2660;(c) S.E. Creutz, K.J. Lotito, G.C. Fu, J.C. Peters, Photoinduced Ullmann C-N coupling:demonstrating the viability of a radical pathway, Science 338(2012) 647-651;(d) C.C.Malakar, A. Baskakova, J. Conrad, U. Beifuss, Copper-catalyzed synthesis of quinazolines in water starting from o-bromobenzylbromides and benzamidines, Chem. Eur. J. 18(2012) 8882-8885;(e) W. Liu, L.Y. Han, R.L. Liu, L.G. Xu, Y.L. Bi, Copper-catalyzed N-arylation of 2-arylindoles with aryl halides, Chin. Chem. Lett. 25(2014) 1240-1243;(f) M. Hamidreza, M.G. Mohammad, Practical copper-catalyzed N-arylation of amines with 20% aqueous solution of n-Bu4NOH, Chin. Chem. Lett. 23(2012) 301-304. |
| [3] | (a) Cu-catalyzed arylation reactions with O-nucleophiles, see:F. Ullmann, P. Sponagel, Ueber die phenylirung von phenolen, Chem. Ber. 38(1905) 2211-2212;(b) A. Ouali, J.F. Spindler, A. Jutand, M. Taillefer, Nitrogen ligands in coppercatalyzed arylation of phenols:structure/activity relationships and applications, Adv. Synth. Catal. 349(2007) 1906-1916;(c) D. Maiti, S.L. Buchwald, Orthogonal Cu- and Pd-based catalyst systems for the O- and N-arylation of aminophenols, J. Am. Chem. Soc. 131(2009) 17423-17429;(d) Y.P. Zhang, A.H. Shi, Y.S. Yang, C.L. Li, Impregnated copper on magnetite as catalyst for the O-arylation of phenols with aryl halides, Chin. Chem. Lett. 25(2014) 141-145. |
| [4] | (a) Cu-catalyzed arylation reactions with S-nucleophiles, see:N. Barbero, R. SanMartin, E. Dominguez, An efficient copper-catalytic system for performing intramolecular O-arylation reactions in aqueous media. New synthesis of xanthones, Green Chem. 11(2009) 830-836;(b) H. Deng, Z. Li, F. Ke, X. Zhou, Cu-catalyzed three-component synthesis of substituted benzothiazoles in water, Chem. Eur. J. 18(2012) 4840-4843. |
| [5] | (a) Cu-catalyzed arylation reactions with activated C-nucleophiles, see:W.R.H. Hurtley, CCXLIV.-replacement of halogen in orthobromo-benzoic acid, J. Chem. Soc. (1929) 1870-1873;(b) T. Minami, T. Isonaka, Y. Okada, J. Ichikawa, Copper(I) salt-mediated arylation of phosphinyl-stabilized carbanions and synthetic application to heterocyclic compounds, J. Org. Chem. 58(2009) 7009-7015;(c) Y. Fang, C. Li, O-arylation versus C-arylation:copper-catalyzed intramolecular coupling of aryl bromides with 1,3-dicarbonyls, J. Org. Chem. 71(2006) 6427-6431. |
| [6] | G. Danoun, A. Tlili, F. Monnier, M. Taillefer, Direct copper-catalyzed a-arylation of benzyl phenyl ketones with aryl iodides:route towards tamoxifen, Angew. Chem. Int. Ed. 51(2012) 12815-12819. |
| [7] | R. Xie, Y. Ling, H. Fu, Copper-catalyzed synthesis of benzocarbazoles via a-Carylation of ketones, Chem. Commun. 48(2012) 12210-12212. |
| [8] | (a) Y. Wang, C. Chen, J. Peng, M. Li, Copper(II)-catalyzed three-component cascade annulation of diaryliodoniums, nitriles, and alkynes:a regioselective synthesis of multiply substituted quinolines, Angew. Chem. Int. Ed. 52(2013) 5323-5327;(b) X. Su, C. Chen, Y. Wang, et al., One-pot synthesis of quinazoline derivatives via [2+2+2]cascade annulation of diaryliodonium salts and two nitriles, Chem. Commun. 49(2013) 6752-6754;(c) F. Wang, C. Chen, G. Deng, C. Xi, Concise approach to benzisothiazol-3(2H)-one via copper-catalyzed tandem reaction of o-bromobenzamide and potassium thiocyanate in water, J. Org. Chem. 77(2012) 4148-4151. |
| [9] | Z. Lou, S. Zhang, C. Chen, et al., Concise synthesis of 1-naphthols under mild conditions through a copper-catalyzed arylation of methyl ketones, Adv. Synth. Catal. 356(2014) 153-159. |
| [10] | (a) K. Okuma, R. Itoyama, A. Sou, N. Nagahora, K. Shioj, Tandem carbon-carbon bond insertion and intramolecular aldol reaction of benzyne with aroylacetones:novel formation of 4,40-disubstituted 1,10-binaphthols, Chem. Commun. 48(2012) 11145-11147;(b) D. Mal, A.K. Jana, P. Mitra, K. Ghosh, Benzannulation for the regiodefined synthesis of 2-alkyl/aryl-1-naphthols:total synthesis of arnottin I, J. Org. Chem. 76(2011) 3392-3398;(c) H. Xu, S. Li, H. Liu, H. Fu, Y. Jiang, Simple and efficient copper-catalyzed cascade synthesis of naphthols containing multifunctional groups under mild conditions, Chem. Commun. 46(2010) 7617-7619;(d) G. Chai, Z. Lu, C. Fu, S. Ma, Studies on the tandem reaction of 4-aryl-2, 3-allenoates with organozinc reagents:a facile route to polysubstituted naphthols, Chem. Eur. J. 15(2009) 11083-11086;(e) A.G. Sergeev, T. Schulz, C. Torborg, et al., Palladium-catalyzed hydroxylation of aryl halides under ambient conditions, Angew. Chem. Int. Ed. 48(2009) 7595-7599;(f) S. Akai, T. Ikawa, S. Takayanagi, et al., Synthesis of biaryl compounds through three-component assembly:ambidentate effect of the tert-butyldimethylsilyl group for regioselective diels-alder and hiyama coupling reactions, Angew. Chem. Int. Ed. 47(2008) 7673-7676;(g) X. Huang, J. Xue, A novel multicomponent reaction of arynes, b-keto sulfones, and Michael-type acceptors:a direct synthesis of polysubstituted naphthols and naphthalenes, J. Org. Chem. 72(2007) 3965-3968;(h) H. Tsukamoto, Y. Kondo, Palladium(II)-catalyzed annulation of alkynes with ortho-ester-containing phenylboronic acids, Org. Lett. 9(2007) 4227-4230;(i) T. Hamura, T. Suzuki, T. Matsumoto, K. Suzuki, Tandem ring expansion of alkenyl benzocyclobutenol derivatives into substituted naphthols, Angew. Chem. Int. Ed. Engl. 118(2006) 6442-6444;(j) X. Zhang, S. Sarkar, R.C. Larock, Synthesis of naphthalenes and 2-naphthols by the electrophilic cyclization of alkynes, J. Org. Chem. 71(2009) 236-243. |
| [11] | R. Abazari, F. Heshmatpour, S. Balalaie, Pt/Pd/Fe trimetallic nanoparticle produced via reverse micelle technique:synthesis, characterization, and its use as an efficient catalyst for reductive hydrodehalogenation of aryl and aliphatic halides under mild conditions, ACS Catal. 3(2013) 139-149. |
| [12] | N. Miyaura, A. Suzuki, Palladium-catalyzed cross-coupling reactions of organoboron compounds, Chem. Rev. 95(1995) 2457-2483. |
| [13] | D. Vuluga, J. Legros, B. Crousse, D. Bonnet-Delpon, Facile access to fluorinated aryl and vinyl ethers through copper-catalysed reaction of fluoro alcohols, Eur. J. Org. Chem. (2009) 3513-3518. |
 2015, Vol.26
2015, Vol.26