Shape-memory polymers (SMPs) are stimuli-responsive materials,which have the capability of recovering their shape upon application of external stimuli such as thermal treatment,light,magnetic or chemicals[1, 2, 3].Compared with shape-memory alloys (SMAs) and shape-memory ceramics (SMCs),SMPs show prominent advantages in high elastic deformation and large recoverable strain,facility of tailoring the architecture,low cost,light weight,and easily processing[4].However,they also[4TD$DIF]exist inevitable disadvantages such as low stiffness,which results in a relatively small recovery force under constraint when faced with large mechanical resistance.In order to overcome this problem,a great deal of stiff nano-fillers has been incorporated into polymer matrices to fabricate SMPCs[5].Gall[6]embedded SiC into shapememory epoxy resin to pursue ceramic enhancement.Expectedly,both the hardness and elastic modulus of the composites increase,but the unconstrained recoverable strain decreases.Actually,the high filler loading will bring some negative[5TD$DIF]effects on the performance of the polymer matrix.The blooming development of the polymer nanocomposites provides an efficient strategy to realize significant improvement of the performance upon a small amount of filler in nanoscale,such as carbon nanotubes (CNTs) and nanoclay[7].Recently,the crystalline aliphatic polyesters such as poly (p-dioxanone)(PPDO)[8]and poly (ε-caprolactone)(PCL) have been utilized to develop the SMPs[9, 10, 11].The previous work of Lendlein[11]demonstrated that PPDO-PCL copolymers display very good shape memory performance with a suitable hard domain (PPDO) and switching domain (PCL) content.Like the most SMPs,it also shows low recovery stress and recovery speed which caused by its low stiff.In present work,we try to introduce some cheap but high efficient stiff filler to reinforce the PPDO-PCL matrix in a well-controlled manner.As we known,the rod-like nanofillers offer better reinforcement than spherical nanoparticles[7].Here,we choose sepiolite (SEP),a kind of fibrous nanoclay with high aspect ratio,as the stiff filler to incorporate into PPDO-PCL matrix.Another fascinating feature of SEP is the high-density Si-OH on its surface[12],which can be utilized as initiating sites for the ring-opening polymerization of lactone such as caprolactone (CL).In this paper,PPDO-PCL/OSEP shape memory nanocomposites with different OSEP content were prepared via chainextending with PPDO and PCL/OSEP precursors.Here,PPDO precursor was synthesized by ring-opening polymerization of pdioxanone (PDO)[13],meanwhile,PCL/OSEP precursor was prepared by surface-initiated polymerization of ε-CL in the presence of OSEP.The dispersion of the OSEP,thermal behavior,mechanical properties of the nanocomposites were investigated,furthermore,the shape memory performance,including fixity and recovery ratio and generated stress during deformation process were also addressed.
2.Experimentalp-dioxanone (PDO) was provided by the pilot plant of the center for the degradable and flame-retardant polymeric materials (Chengdu,China),ε-caprolactone (ε-CL) was purchased from Sigma-Aldrich.Both PDO and ε-CL were distilled under reduced pressure until the water content was less than 50 ppm.Stannous octoate (Sn(Oct)2)(Sigma-Aldrich) was used as received.After being diluted with dry toluene,the Sn(Oct)2 solution was stored in glass ampoule under nitrogen.The solvents (chloroform (CHCl3) and 1,2-dichloroethane (C2H4Cl2)) were supplied by Kelong Reagent Corp.,which were refluxed and dehydrated by CaH2 and distilled before use.Sepiolite (SEP) was supplied by Tolsa SA (Spain),the preparation of organic modified SEP (OSEP) is followed by our previous work[14].All other chemicals were analytical grade reagents and used as purchased.
The preparation of PPDO-diol,PCL-diol and PCL/OSEP precursors was introduced in supporting information (the synthetic route was illustrated in scheme S1 in supporting information,and the feed ratios,molecular weights and their distributions were listed in Table S1 in supporting information).The PPDO-PCL/OSEP nanocomposites were prepared by coupling PPDO-diol and PCL/OSEP precursors with hexamethylene diisocyanate (HDI) in C2H4Cl2 at 75 ℃ in accordance with Niu et al.[15].Firstly,PPDO-diol and PCL/OSEP precursors were put into a reaction flask and dehydrated at 75 ℃ under vacuum for 3 h.Secondly,dry C2H4Cl2 was injected into the flask to dissolve the precursors with stirring for about 30 min,and a predetermined amount of HDI was injected to actuate the coupling reaction.After 3 h of pre-reaction,at last,the mixture was precipitated in excessive hexane.After the precipitates were completely dry,they were hot-pressed at 140 ℃ for various tests.
The 1H NMR spectra were recorded at room temperature with Bruker AV400 spectrometers (Bruker,Germany) at 400 MHz in CDCl3,and the chemical shifts were calibrated to TMS.The FT-IR spectra were performed with a Nicolet 670 FT-IR spectrometer (Thermo Nicolet,USA) for a scanning coverage from 4000 cm-1 to 400 cm-1 at a spectral resolution of 4 cm-1 using 32 scans.The molecular weight of precursors and PPDO-PCL/OSEP nanocomposites were analyzed by GPC using a Waters instrument equipped with a model 1515 pump,a Waters model 717 autosampler,and a 2414 refractive index detector with the flowing rate of chloroform is 1.0 mL/min,and the concentration of sample was about 2.5 mg/mL.All quantitative shape memory cycles were measured under tension using the DMA Q800 under a controlled-force mode according to previous literature reports[16].The detail of the test procedure were found in Supporting information,the values of shape fixity ratio (Rf) and shape recovery ratio (Rr) were determined according to the equation S(1) and S(2) list in Supporting information.
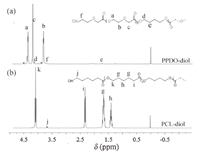
3.Results and discussionThe 1H NMR spectra of PPDO-diol and PCL-diol were illustrated in Fig.1.In the spectrum of PPDO-diol (Fig.1a),three signals with high intensity at 4.33 ppm (δ Ha),3.78 ppm (δ Hb) and 4.18 ppm (δ Hc) are attributed to the three methylene protons in repeat units of PPDO-diol,respectively.In addition,the weak signal at 3.69 ppm (δ Hf) is assigned to the corresponding proton in terminal group of PPDO-diol.The spectrum of PCL-diol is shown in Fig.1b,four signals with high intensity at 4.06 ppm (δ Hk),2.30 ppm (δ Hi),1.68 ppm (δ Hg) and 1.37 ppm (δ Hh) are attributed to the fourmethylene protons in repeat units of PCL-diol,respectively.Moreover,the weak signal at 3.72 ppm (δ Hj) is assigned to the corresponding proton in terminal group of PCL-diol.
|
Download:
|
| Fig. 1.1H NMR spectra of dihydroxyl-terminated PPDO-diol prepolymer (a) and dihydroxyl-terminated PCL-diol prepolymer (b). | |
Fig.2 shows FT-IR spectra of OSEP (a),neat PCL-diol (b),and sepiolite isolated from PCL/OSEP3 precursor (c),respectively.The characteristic peaks of PCL-diol are observed in the isolated sepiolite at 1749 cm-1(C55O stretching) and 2927 cm-1(C=0 stretching),which proved that there has a part of PCL segments grafted on OSEP.

|
Download:
|
| Fig. 2.FT-IR spectra of OSEP (a), neat PCL-diol (b) and OSEP isolated from PCL/OSEP nanocomposites (c). | |
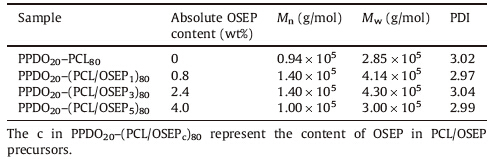
PPDO-PCL multiblock copolymer and PPDO-PCL/OSEP nanocomposites were fabricated by coupling PPDO-diol with PCL-diol or PCL/OSEP precursors using HDI,the detail information of products are displayed in Table 1.Since the multiblock copolymer with the composition of PPDO/PCL=20/80 exhibits good shapememory performance,we choose it as the matrix to fabricate nanocomposites.
| Table 1 The feed ratios and molecular weights of PPDO–PCL/OSEP nanocomposites. |
It is well known that the dispersion of the nanofiller in polymer matrix significantly affects the properties of nanocomposites[17].Fig.3a-c shows the SEM photographs of the fracture surfaces of composites with different OSEP loadings.It was found that OSEP fibers distributed uniformly in the sample with low OSEP content,such as PPDO20-(PCL/OSEP1)80(a) and PPDO20-(PCL/OSEP3)80(b),but aggregation could be observed in PPDO20-(PCL/OSEP5)80(Fig.3c),which owns highest OSEP content.TEM observation was also employed for PPDO20-(PCL/OSEP3)80,and it provides asolid demonstration of fine dispersion of OSEP fibers in nanoscale (Fig.2b1 and b2).

|
Download:
|
| Fig. 3.SEM images (10 kV,20,000×) of PPDO20-(PCL/OSEP1)80(a),PPDO20-(PCL/OSEP3)80(b),PPDO20-(PCL/OSEP5)80(c),and TEM images of PPDO20-(PCL/OSEP3)80(b1 and b2). | |
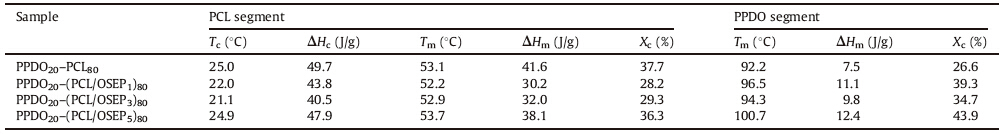
For a thermally-induced SMP based on the crystalline and melting transition,the thermal behavior of the samples plays a dominant role both on the programming setting and the final performance of SME.Therefore,the DSC analysis has been conducted to disclose the detailed information about the thermal properties of PPDO-PCL/OSEP nanocomposites.Fig.4 illustrates the DSC traces for PPDO-PCL multiblock copolymers and PPDO-PCL/OSEP nanocomposites,and all the relevant data were summarized in Table 2.It reveals that both PPDO and PCL segments show individual melt peaks,which allow they act as hard segment and soft segment,respectively.Moreover,the mechanical properties were also tested,and obviously enhancement was achieved by incorporating OSEP nanofiber (Table 3).

|
Download:
|
| Fig. 4.The DSC scans of samples at 5 ℃/min: (a) the cooling scans and (b) the subsequent heating scans. | |
| Table 2 Relevant data from DSC curves of PPDO–PCL/OSEP composites. |
| Table 3 The mechanical properties and shape-memory effects of PPDO–PCL/OSEP nanocomposites. |
Cyclic thermal mechanical test was conducted by DMA to evaluate the shape-memory performance of samples,the cyclic curves were illustrated in Fig.S1,the results were also summarized in Table 3.Delightedly,the nanocomposites with fine dispersion of OSEP exhibit excellent fixing and recover ability with the Rf and Rr values higher than 99%.When OSEP loading is higher than 5%,a slightly decrease in Rr was observed.Fig.5a displays the recovery process of PPDO20-(PCL/OSEP3)80 in about 1 min recorded by digital camera.In view of the purpose in this work,the recovery stress is the key point we concerned.Fig.5b illustrates the influence of the content of OSEP on the generated stress under deformation process,which corresponding to the recovery stress directly.It proves that incorporating OSEP with fine dispersion significantly increase the generated stress under deformation process,which may attribute to the enhancement of the molecular interactions between the matrix and OSEP.This positive effect[16TD$DIF]is debased when the content of OSEP reaches 5%,which may be caused by the appearance of nanofibers'aggregation.A schematic representation[17TD$DIF]of the molecular mechanism was illustrated in Fig.5c,not only the physical crosslink formed by crystalline of PPDO segments but also the slight crosslink formed by OSEP in PCL domain may serve as the netpoints in shape-memory nanocomposites.

|
Download:
|
| Fig. 5.(a)The digital photos of the recovery process of PPDO20-(PCL/OSEP3)80.(b) The influence of OSEP on the generated stress of PPDO20-(PCL/OSEP)80.(c) Schematic representation of the molecular mechanism of the slight crosslinking caused by OSEP grafted PCL polymer chains in SME process. | |
Shape-memory poly (p-dioxanone)-poly (ε-caprolactone)/sepiolite (PPDO-PCL/OSEP) nanocomposites were fabricated by chainextending the PPDO-diol and PCL/OSEP precursors.SEM and TEM demonstrate that the composites display a fine dispersion while the OSEP loading was lower that 5%.The SME test reveals that the PPDO-PCL/OSEP nanocomposites exhibit good shape-memory performance and enhanced recovery stress.
AcknowledgmentThis work was supported financially by the National Science Foundation of China (Nos.51473096,51421061,J1103315) and Program for Changjiang Scholars and Innovative Research Team in University[18TD$DIF]of Ministry of Education of China (No.IRT 1026).
Appendix A.Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.07.019.
| [1] | R. Xiao, J.K. Guo, D.L. Safranski, T.D. Nquyen, Solvent-driven temperature memory and multiple shape memory effects, Soft Matter. 11(2015) 3977-3985. |
| [2] | Y.F. Luo, M.N. Huang, S.J. Wang, Y. Fu, Y.L. Wang, Design, synthesis and characterization of novel poly (urethane-urea) based on a macrodiol from poly (lactic acid) and poly (p-dioxanone), Chin. Chem. Lett. 22(2011) 237- 240. |
| [3] | X.T. Zheng, S.B. Zhou, Y. Xiao, et al., Shape memory effect of poly (D,L-lactide)/Fe3O4 nanocomposites by inductive heating of magnetite particles, Colloids Surf. B:Biointerfaces 71(2009) 67-72. |
| [4] | W.M. Huang, Z. Ding, C.C. Wang, et al., Shape memory materials, Mater. Today 13(2010) 54-61. |
| [5] | C.L. Huang, M.J. He, M. Huo, et al., A facile method to produce PBS-PEG/CNTs nanocomposites with controllable electro-induced shape memory effect, Polym. Chem. 4(2013) 3987-3997. |
| [6] | K. Gall, M.L. Dunn, Y.P. Liu, et al., Shape memory polymer nanocomposites, Acta Mater. 50(2002) 5115-5126. |
| [7] | B. Xu, Y.Q. Fu, M. Ahmad, et al., Thermo-mechanical properties of polystyrenebased shape memory nanocomposites, J. Mater. Chem. 20(2010) 3442-3448. |
| [8] | K.K. Yang, X.L. Wang, Y.Z. Wang, Poly (p-dioxanone) and its copolymers, J. Macromol. Sci. Part C:Polym. Rev. 42(2002) 373-398. |
| [9] | L.P. Xiao, M. Wei, M.Q. Zhan, et al., Novel triple-shape PCU/PPDO interpenetrating polymer networks constructed by self-complementary quadruple hydrogen bonding and covalent bonding, Polym. Chem. 5(2014) 2231-2241. |
| [10] | M. Wei, M.Q. Zhan, D.Q. Yu, et al., A novel poly (tetramethylene ether) glycol and poly (ε-caprolactone) based dynamic network via quadruple hydrogenbonding with triple-shape effect and self-healing capacity, ACS Appl. Mater. Interfaces 7(2015) 2585-2596. |
| [11] | A. Lendlein, R. Langer, Biodegradable, elastic shape-memory polymers for potential biomedical applications, Science 296(2002) 1673-1676. |
| [12] | H. Shariatmadari, A.R. Mermut, Magnesium- and silicon-induced phosphate desorption in smectite-, palygorskite-, and sepiolite-calcite systems, Soil Sci. Soc. Am. J. 63(1999) 1167-1173. |
| [13] | B. Wang, C. Ma, Z.C. Xiong, et al., Synthesis of novel copolymer:poly (p-dioxanone-co-L-phenylalanine), Chin. Chem. Lett. 24(2013) 392-396. |
| [14] | Z.C. Qiu, J.J. Zhang, Y. Niu, et al., Preparation of poly (p-dioxanone)/sepiolite nanocomposites with excellent strength/toughness balance via surface-initiated polymerization, Ind. Eng. Chem. Res. 50(2011) 10006-10016. |
| [15] | Y. Niu, P. Zhang, J.J. Zhang, et al., Poly (p-dioxanone)-poly (ethylene glycol) network:synthesis, characterization, and its shape memory effect, Polym. Chem. 3(2012) 2508-2516. |
| [16] | T. Xie, Tunable polymer multi-shape memory effect, Nature 464(2010) 267-270. |
| [17] | Z.Q. Duan, Y.T. Liu, X.M. Xie, X.Y. Ye, A simple and green route to transparent boron nitride/PVA nanocomposites with significantly improved mechanical and thermal properties, Chin. Chem. Lett. 24(2013) 17-19. |
 2015, Vol.26
2015, Vol.26